Vinylations of organic compounds are useful synthetic transformations. Coupling nucleophiles with vinyl halides can introduce β,γ-unsaturated groups in an efficient way. However, transition-metal catalyzed reactions of this type have been limited mostly to enolate and nitronate substrates.
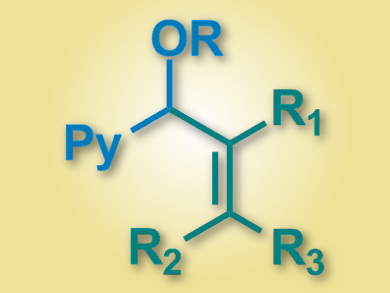
Xiaodong Yang, Patrick J. Walsh, and colleagues, University of Pennsylvania, Philadelphia, USA, have developed a palladium-catalyzed α-alkenylation of pyridylmethyl ethers with vinyl bromides (product pictured). The team uses a Pd–NIXANTPHOS catalyst (NIXANTPHOS = 4,6-bis(diphenylphosphino)phenoxazine), together with LiN(SiMe3)2 as a base and cyclopentyl methyl ether (CPME) as a solvent.
The reaction proceeded under mild conditions (65 °C over 12 hours), and the team was able to couple a variety of pyridylmethyl ethers with a range of vinyl bromides. The yields were good to excellent (up to 97 %). The researchers are working on a catalyst for an enantioselective variant of the reaction.
- Palladium-Catalyzed Selective α-Alkenylation of Pyridylmethyl Ethers with Vinyl Bromides,
Xiaodong Yang, Byeong-Seon Kim, Minyan Li, Patrick J. Walsh,
Org. Lett. 2016.
DOI: 10.1021/acs.orglett.6b00815