Zero-valent main group compounds are difficult to stabilize and thus an interesting challenge for inorganic chemists. There are a number of stable low-valent p-block complexes with unusual reactivities, often similar to transition metal complexes. Neutral zero-valent s-block compounds, however, have been predicted by computational chemists, but have never been synthesized until now.
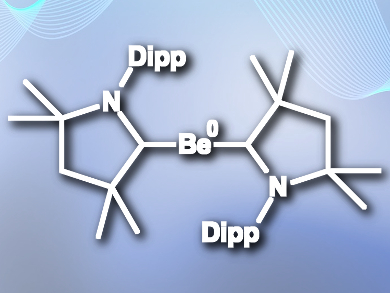
Holger Braunschweig, University of Würzburg, Germany, and colleagues have isolated the first zero-valent neutral beryllium complexes (example pictured), stabilized by cyclic (alkyl)(amino)carbene (CAAC) ligands. The team added a single ligand to BeCl2 in benzene solution to give (CAAC)BeCl2, and obtained the final product, [Be(CAAC)2], by reducing the beryllium with KC8 in the presence of a second equivalent of CAAC ligand.
The researchers characterized the compound using X-ray crystallography, 9Be NMR, 1H NMR, and UV-Vis spectroscopy. The complexes have very short beryllium-carbon bond lengths and linear beryllium coordination, which indicates strong multiple Be–C bonding. Density functional theory (DFT) calculations confirm this and indicate a strong three-centre two-electron π-bond across the C–Be–C unit, further stabilized through π-backdonation from beryllium to the ligands.
- Neutral zero-valent s-block complexes with strong multiple bonding,
Merle Arrowsmith, Holger Braunschweig, Mehmet Ali Celik, Theresa Dellermann, Rian D. Dewhurst, William C. Ewing, Kai Hammond, Thomas Kramer, Ivo Krummenacher, Jan Mies, Krzysztof Radacki, Julia K. Schuster,
Nat. Chem. 2016.
DOI: 10.1038/nchem.2542