Title: C(sp²)–H Bond Activation with a Heterometallic Nickel—Aluminium Complex
Authors: Joseph A. Zurakowski, Benedek Stadler, Marcus W. Drover, Mark Richard Crimmin
Published: 10 July 2025 in Angewandte Chemie
🔬 What They Did
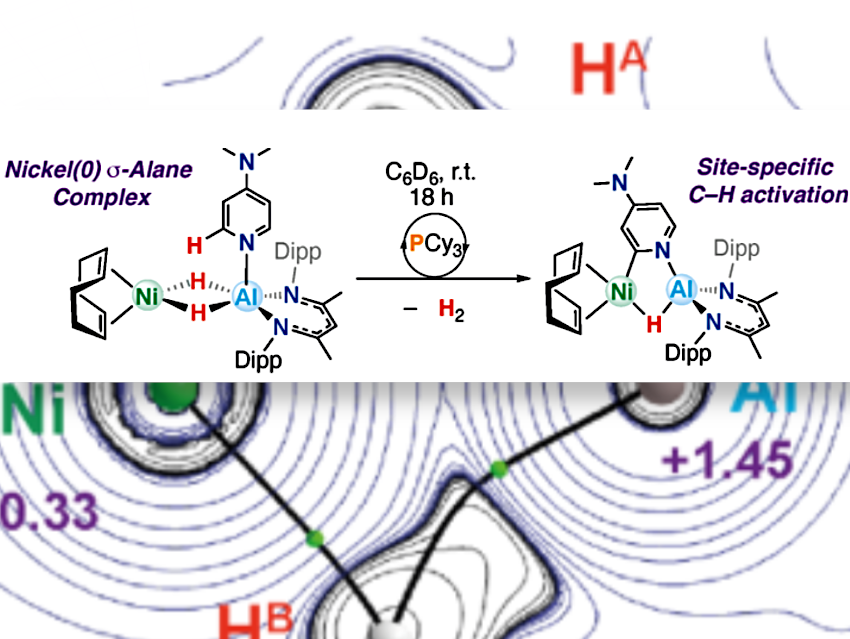
The researchers synthesized a nickel–aluminium (Ni–Al) complex that can selectively activate the C–H bond of 4-dimethylaminopyridine (DMAP) or pyridine at the 2-position.
The reactive complex forms readily from simple precursors, [Ni(COD)₂] (COD = 1,5-cyclooctadiene) and [(BDI)AlH₂] (BDI = 2,6-diisopropylphenyl-β-methyldiketiminate), yielding [(η⁴-COD)Ni(μ-H)₂Al(BDI)].
This C–H bond activation reaction is catalyzed by a simple phosphine, PCy₃, which gives faster rates and higher yields of C–H activation. Pictured above; with Dipp = 2,6-di-isopropylphenyl.
🔍 What They Found?
The Ni–Al complex activates C(sp²)–H bonds by oxidative addition at the 2-position of DMAP or pyridine, followed by H₂ release. The room temperature, multistep reaction is driven by the cooperative action of both metals and is controlled by how supporting ligands exchange at the nickel site.
The Al centre plays a key role in anchoring the substrate in place through a Lewis-acid/
Lewis-base interaction. PCy₃ catalyses the oxidative addition step through ligand exchange at the Ni site and stabilization of the key transition state through its strong σ-donor properties.
🌍 Why It Matters
For some time now, nickel and aluminum pairings have been shown to facilitate the C-H bond activation and functionalization of heterocycles, but mechanistic information has lagged. This work shows precisely how combining nickel with a Lewis-acidic metal like aluminium enables more precise and efficient C–H fictionalization. This is important for building complex molecules in fewer steps.
It also highlights that controlling ligand exchange may be the key determinant of catalytic reaction rates in Ni–Al systems, guiding future catalyst design.
🧩 Cool Detail
The aluminium doesn’t just sit idle. It acts like a molecular anchor, holding the pyridine in place while nickel breaks the C–H bond.
DFT calculations indicate that both C(sp²)–H bond activation and H₂ reductive elimination in the Ni–Al system are low-energy, non-rate-limiting steps. The rate-determining step is likely the ligand exchange at nickel from COD to PCy₃.