Title: Synthesis of the Soft Lewis Superacid Tris(4-bromo-2,3,5,6-tetrafluorophenyl)borane B(C₆F₄Br)₃ via C‒Ag to C‒B Transmetalation
Authors: Amina L. Moshtaha, Erika M. Peter, Robin Sievers, Tim-Niclas Streit, Moritz Malischewski
Published: 13 September 2025 in Chemistry – A European Journal
🔬 What They Did
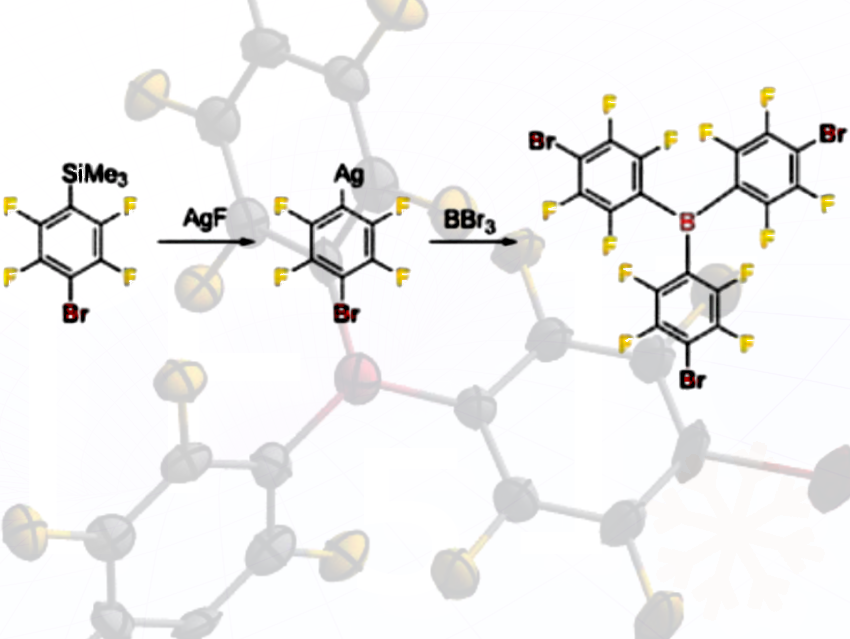
- The researchers synthesized a new bromine-substituted triarylborane, B(C₆F₄Br)₃, using a silver-to-boron “transmetalation” step—a route not previously used for this family of Lewis acids.
- They first prepared the silver aryl precursor AgC₆F₄Br, determined its crystal structure, and then transferred the aryl groups to boron to obtain the borane.
🔍 What They Found?
- The new borane is a soft Lewis superacid, about 1–2% more acidic than the well-known benchmark B(C₆F₅)₃, as shown by computational (FIA/HIA) and experimental (Gutmann–Beckett) methods.
- The heavy bromine atoms are expected to make single-crystal X-ray analysis of its adducts easier, broadening opportunities to study Lewis acid–base complexes.
🌍 Why It Matters
Expanding the library of highly acidic, perfluorinated triarylboranes provides stronger catalysts and new building blocks for materials chemistry, where precise structural analysis is essential.
🧩 Cool Detail
The silver compound AgC₆F₄Br crystallized as a tetrameric toluene solvate ([2(AgC₆F₄Br)₄•7.46(toluene)]), as determined by single-crystal X-ray diffraction.
- Four AgC₆F₄Br units associate to form a tetramer, which is unusual for silver aryl compounds.
- The tetramer crystallizes with approximately 7.46 toluene molecules per tetramer, showing that the solvent is incorporated into the crystal lattice.
- This tetramer serves as the key intermediate in the transmetalation to boron, enabling the synthesis of the new Lewis acid B(C₆F₄Br)₃.