Title: A Shape-Complementarity Approach to Inducing Short Au(III)···Au(III) Contacts in Tetracyanoaurate(III) Salts
Authors: Thomas E. Karpiuk, Robby Y. Williams-Sekiguchi, Samyadeb Mahato, Tim Storr, Daniel B. Leznoff
Published: 12 June 2025 in Chemistry – A European Journal
🔍 What They Did?
Daniel B. Leznoff, Simon Fraser University, Burnaby, BC, Canada, and colleagues have explored how the shape of cations can influence how gold-based building blocks come together in a solid structure.
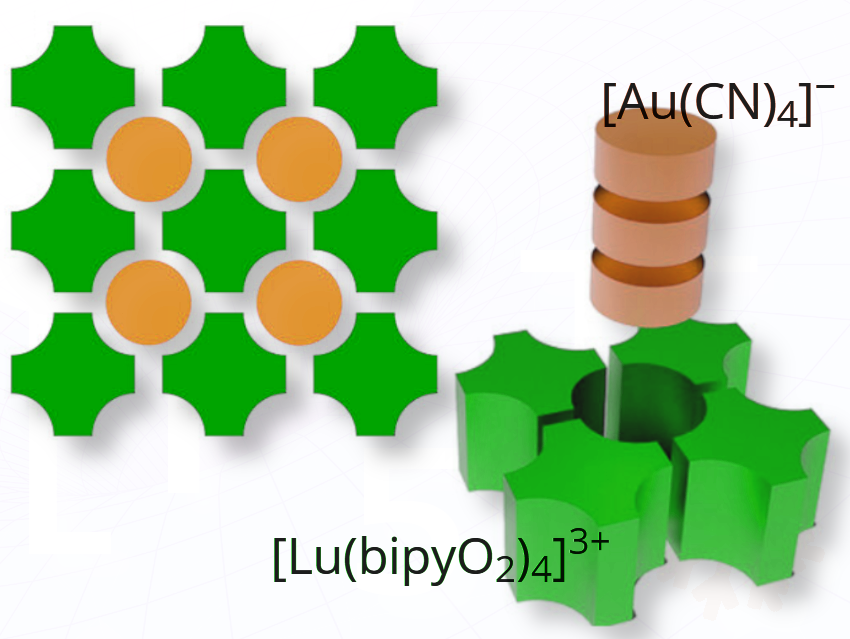
They synthesized and characterized 16 gold(III) tetracyanide salts with various cation shapes to study how shape influences crystal packing and Au(III)···Au(III) interactions.
They used crystallography, Hirshfeld surface analysis, and quantum chemical calculations to probe structure and aurophilic behavior.
🔍 What They Found
By carefully choosing the shape of the cation, the researchers were able to control the packing of gold-containing anions, sometimes enabling direct gold–gold (Au···Au) contacts, known as aurophilic interactions.
Au(III)···Au(III) aurophilic interactions were observed only when the cation and anion had complementary shapes, enabling tight packing—most notably in the RE(bipyO₂)₄Au₃ (RE = Sc, Y, La) series.
The shortest Au(III)···Au(III) contact ever reported (3.3351 Å) was measured in these structures.
🌍 Why It Matters
Demonstrates how shape complementarity can be a predictive tool for designing inorganic crystals with specific packing motifs and weak noncovalent interactions.
Offers a generalizable strategy for building isoreticular frameworks, relevant to MOFs, host–guest systems, and functional materials.
🧩 Cool Detail
The rare-earth–gold structure behaves like a molecular tiling template: it directs how nearby anions align, leaving “cavities” that guide and stabilize unexpected close contacts—like puzzle pieces snapping into place.