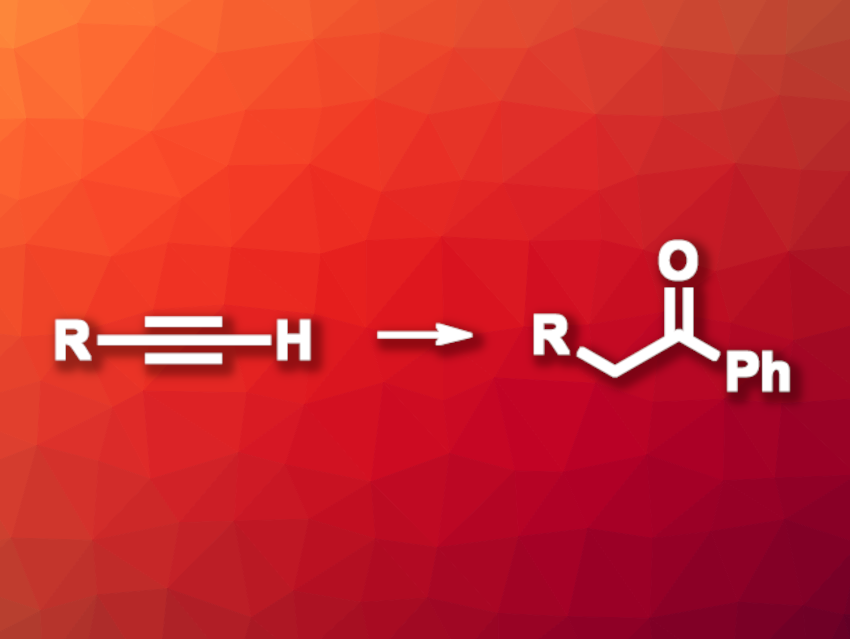
Ketones are useful products and intermediates in organic synthesis. They can be prepared, for example, by the oxyfunctionalization of alkynes. This type of reaction using terminal alkynes usually features 2,1-regioselectivity, while 1,1-oxyfunctionalization is rare.
Jinglong Chen, Fuzhou University, Fujian, China, Qiuling Song, Fujian Province University and Henan Normal University, China, and colleagues have developed a method for the 1,1-oxycarbonation of terminal alkynes to give ketones. The reaction proceeds via sequential borylation, 1,2-carbon migration, and oxidation with Oxone (2 KHSO5·KHSO4·K2SO4). The team first reacted terminal alkynes with nBuLi, Py·BPh3, and NMe4Cl to obtain alkynyl tetracoordinate boron species. These intermediates were then reacted with Oxone in a mixture of acetone and water to obtain the desired ketones via proton-induced 1,2-carbon migration and oxidation.
Under these conditions, the researchers obtained the target ketones in mostly moderate to good yields and with good functional group tolerance. They scaled up an example synthesis to 5 mmol and obtained a yield of 75 %. The products can be useful substrates for further functionalizations. Overall, the work provides an approach to the regioselective 1,1-oxycarbonation of terminal alkynes.
- 1,1-Oxycarbonation of Terminal Alkynes via Sequential Borylation, 1,2-Migration, and Oxidation with Oxone,
Guan Zhang, Bofan Feng, Yutong Wang, Jinglong Chen, Xingxing Ma, Qiuling Song,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00738