Intramembrane serine proteases (also known as rhomboid proteases) are enzymes that have their catalytic sides embedded in the lipid bilayer of a membrane. They are attractive drug targets, as they are involved in a variety of human diseases. They attack their substrates, which are generally membrane proteins, via an active site that features a serine-histidine pair.
There are various covalent electrophilic scaffolds for rhomboid inhibition. β-Lactams are also interesting in this context. However, for rhomboid proteases, the lactam scaffold has been generally limited to examples with electron-withdrawing substituents on the nitrogen atom so far.
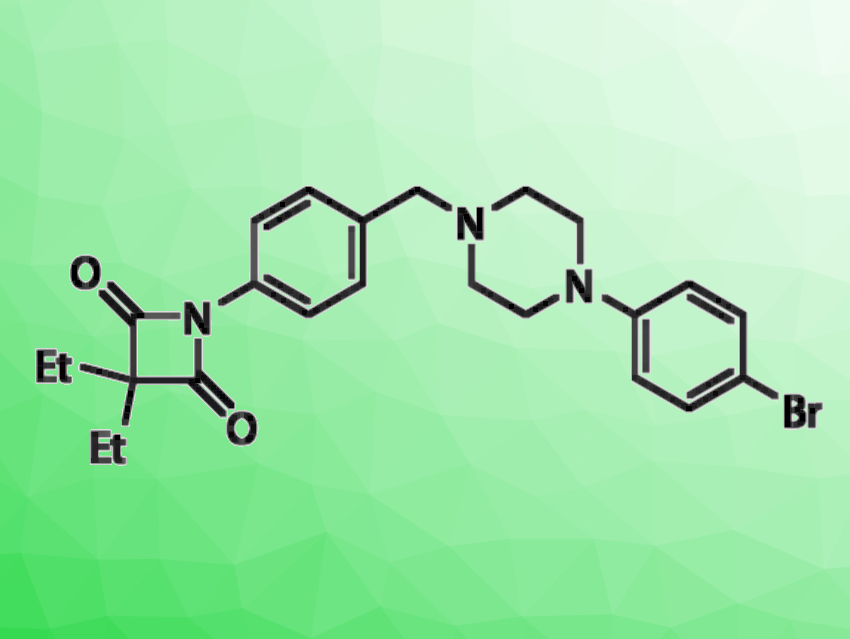
Steven H. L. Verhelst, KU Leuven, Belgium, Leibniz Institut für Analytische Wissenschaften – ISAS – e.V, Dortmund, Germany, and colleagues have investigated whether an oxo-substituent in the lactam ring could make β-lactams suitable for rhomboid protease inhibition, as well. The team discovered 4-oxo-β-lactams (example pictured) that can serve as rhomboid protease inhibitors. They screened a set of these lactams against the Escherichia coli rhomboid GlpG and found that the most potent hits inhibited this protease with submicromolar apparent IC50 values.
The researchers found that the 4-oxo-β-lactams are covalent inhibitors with a reversible mechanism: After acylation of the active site serine, the formed ester bond is only slowly hydrolyzed over time. Overall, the 4-oxo-β-lactam scaffold could provide possibilities to develop more effective inhibitors for this class of proteases.
- 4‐Oxo‐β‐Lactams as Novel Inhibitors for Rhomboid Proteases,
Jian Yang, Luís A. R. Carvalho, Shanping Ji, Suyuan Chen, Rui Moreira, Steven H. L. Verhelst,
ChemBioChem 2023.
https://doi.org/10.1002/cbic.202300418