William R. Dichtel, Northwestern University, Evanston, IL, USA, and coleagues have attached redox-driven artificial ring-threading molecular pumps (AMPs) to polymeric micelles to actively push charged ring-shaped molecules (CBPQT⁴⁺; cyclobis(paraquat-p-phenylene)) from water into the micelle interiors.
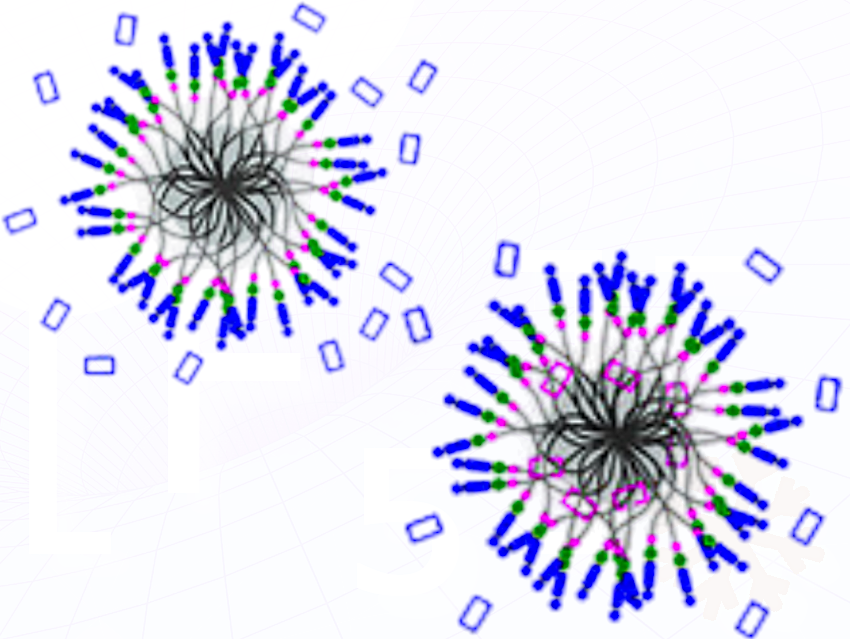
The pump cassette contains a disubstituted bipyridinium recognition site (BIPY²⁺; shown as blue-filled boxes in the figure) linked by a bismethylene bridge to a 2,6-dimethylpyridinium (PY⁺; blue dots) terminal and by a methylene group to an isopropylphenylene (IPP) steric barrier (green dots in the figure).
The AMPs function as redox-driven molecular pumps that transfer CBPQT⁴⁺ rings (blue-outlined boxes in the figure) onto collecting chains through a bipyridinium (BIPY²⁺) recognition site flanked by a steric isopropylphenylene (IPP) barrier . Reduction induces trisradical tricationic host–guest complexation, while subsequent oxidation removes radical bonding and drives ring displacement over the IPP barrier, trapping the rings mechanically in a metastable state even in aqueous media when hydrophilic counterions (chloride ions and trifluoroacetate ions) are used instead of hydrophobic PF₆⁻ counterions.
Repetition of the reduction–complexation → oxidation–ejection sequence constitutes an energy-ratchet: input of redox energy biases each cycle in the same direction, allowing stepwise accumulation of rings on the collecting chain (red-outlined boxes in the figure).
The team found that when the artificial molecular pumps (AMPs) were attached to polymeric micelles, they successfully threaded 65% of the ring molecules (CBPQT⁴⁺) into the micelle interiors. In contrast, the same pumps operating freely in solution (without micelles) threaded 91% of rings. The lower efficiency in micelles likely comes from crowding or competition between molecules inside the micelle environment, which can slow the threading process. This result shows that AMPs don’t just work in simple solutions but they can actively control the composition inside complex water-based structures like micelles.
The work demonstrates that synthetic pumps can mimic natural transmembrane transporters, which actively concentrate molecules in cells. The pumps are tiny, about 860 Da, compared with natural membrane transporters, yet they can perform directional, active transport usually reserved for much larger biological machines. The pump uses controlled electron changes to push the ring in one direction and lock it onto the chain, creating non-equilibrium storage.
The ability to actively concentrate hydrophilic molecules in defined compartments is potentially useful for drug delivery, chemical storage, or nanotechnology.
- Active Transport of Macrocycles into Micelles Using Molecular Pumps
James S. W. Seale, Swagat Sharma, Christopher K. Lee, Han Han, Tyler Jaynes, Eric W. Roth, Saman Shafie, Prof. Yunyan Qiu, Luke Malaisrie, Madison I. Bardot, Long Zhang, Yi-Kang Xing, Dong Jun Kim, Samuel I. Stupp, R. Dean Astumian, Evan A. Scott, William R. Dichtel, J. Fraser Stoddart
Angew. Chem. Int. Ed. 2025
https://doi.org/10.1002/anie.202512899