Johannes Voigtland and Moritz Ludwig, Technical University of Munich, Germany, and their co-authors show how main-group elements like aluminum and silicon can fine-tune transition-metal reactivity by forming stable Al–Si–M (M = Cu, Ag, Au) cores capable of capturing CO₂ and deepen our understanding of silicon’s interactions with coinage metals.
What did you do?
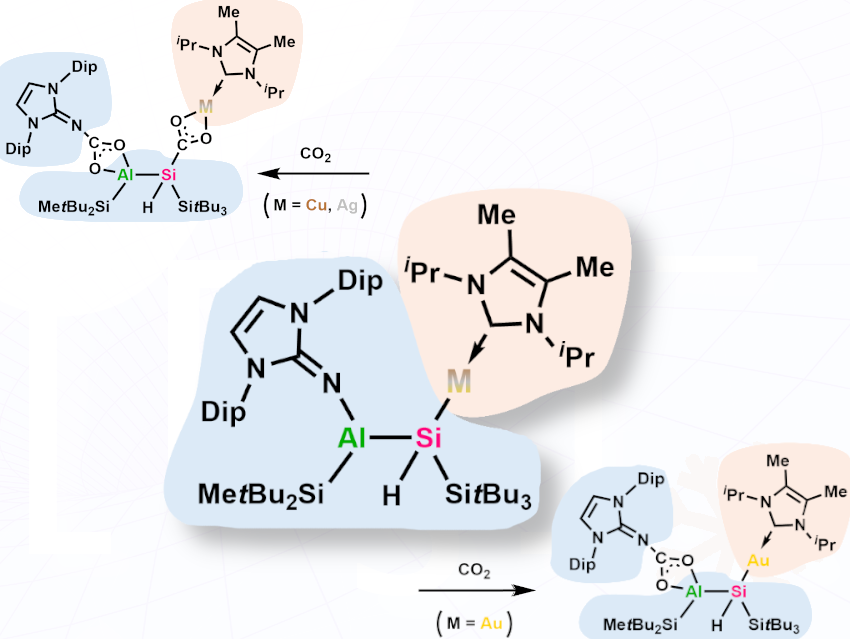
We have employed sodium silanides Na⁺[R₂R’Si–], functionalized with an alumanyl substituent (R’ = AlR₂), to tune the reactivity of coinage metals (M = Cu, Ag, Au). This straightforward synthesis gives alumanyl silyl coinage metal complexes characterized by an Al–Si–M core in high-yields. Experimentally, we investigated the stability of the formed Si–M bonds via regioselective CO₂ activation.
Why are you interested in this?
Our goal was to enhance the reactivity of coinage metal complexes using a ligand system based on aluminum and silicon. Thus, combining main group and transition metal chemistry with the potential for cooperative reactivity.
What is new and cool about this?
Studies on silyl coinage metal complexes are rare and the bonding situation in such complexes is often analyzed via computational methods rather than experimental evidence.
We could systematically show how the stability of the Si–M bond increases descending the group. As a result, the copper and silver analogues are able to insert one equivalent of CO₂ into the Si–M bond. In contrast, the gold center is stronger bound to the silyl group, inhibiting the regioselective insertion of CO₂.
What is the main significance of your results?
Silanides are well suited precursors to tune the reactivity of coinage metals. In our case the adjacent aluminum center additionally stabilizes the nucleophilic silicon centre allowing for the controlled activation of CO₂ by copper and silver silyl complexes.
What is the longer-term vision of your research?
Our unprecedented alumanyl silyl coinage metal complexes demonstrate the ability to regioselectively insert carbon dioxide, establishing a powerful platform for subsequent reactivity. The next step would be to functionalize the encapsulated CO₂ moiety by means of hydration, hydrosilylation, or hydroboration to convert the latter into useful chemicals.
In the long run our dream would be a catalytic system based on a silyl-substituted transition metal center. We hope that our findings might fuel the search for such systems.
What part of your work was the most challenging?
We are used to working with highly air- and moisture-sensitive complexes. Adding light sensitivity to the mix by working with silver and gold-based compounds resulted in some night shifts in the lab.
Do you have any remarks you’d like to share?
We gratefully acknowledge all those whose efforts and dedication made this research possible, and who have contributed to advancing this fascinating and significant field at the interface of main-group and transition-metal chemistry.
Thank you very much for sharing these insights.
The paper they talked about:
- Synthesis of Alumanyl Silyl Coinage Metal Complexes and their Reactivity Toward CO₂,
Johannes Voigtland, Moritz Ludwig, Sebastian Stigler, Shigeyoshi Inoue,
ChemistryEurope 2025.
https://doi.org/10.1002/ceur.202500216
Johannes Voigtland and Moritz Ludwig are Ph.D. students in the group of Professor Shigeyoshi Inoue at the TUM School of Natural Sciences, Department of Chemistry, Catalysis Research Center, and Institute of Silicon Chemistry at the Technical University of Munich in Garching bei München, Germany.
Also of Interest