Title: Aziridination of Olefins by a Copper Phenanthroline Catalyst
Authors: Onnicha Khaikate, Kathrin Strunk, Chiara Cabrele, Nattawut Hassa, Chutima Kuhakarn, Oliver Reiser
Published: 25 September 2025 in Chemistry – A European Journal
🔬 What They Did
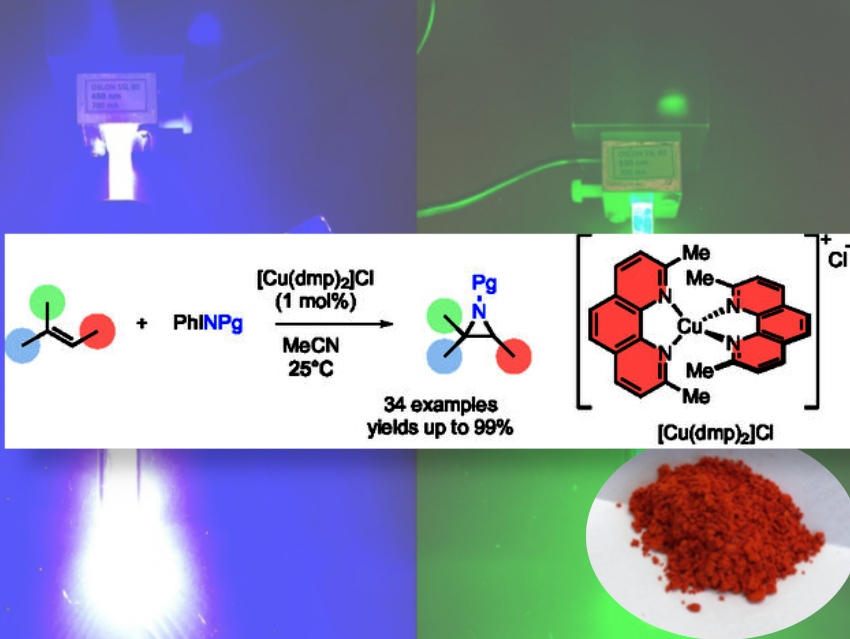
They synthesized the commonly used photoredox catalyst, the copper (I) complex [Cu(dmp)₂]Cl (dmp = 2,9-dimethyl-1,10-phenanthroline), and used it to add a nitrogen atom across carbon–carbon double bonds, forming three-membered aziridine rings quickly and at room temperature without light or heat. In doing so, they established a rapid and efficient [Cu(dmp)₂]Cl-catalyzed aziridination of alkenes with iminoiodinanes.
🔍 What They Found?
The copper catalyst is suitable for a wide range of alkene substrates and N-protected iminoiodinanes (34 examples, up to 99% yield). Especially styrenes and electron-rich 1,3-dienes are found to be suitable substrates, allowing short reaction times (10 min) at low catalyst loading (1 mol%).
Acting as a dual-function catalyst, [Cu(dmp)₂]Cl enables fast aziridination in the dark and, under visible light, promotes further ring-opening reactions to make functionalized amines. This dual role of [Cu(dmp)₂]Cl as both an aziridination and photoredox catalyst highlights its utility for the streamlined synthesis of complex nitrogen-containing scaffolds.
🌍 Why It Matters
This provides a low-cost, versatile route to nitrogen-rich molecules that are key building blocks for pharmaceuticals and materials, expanding options beyond expensive ruthenium photocatalysts.
🧩 Cool Detail
The copper (I) complex is bright red as shown in the picture and remains active even sitting on the lab bench, making the reaction setup as simple as mixing and stirring.