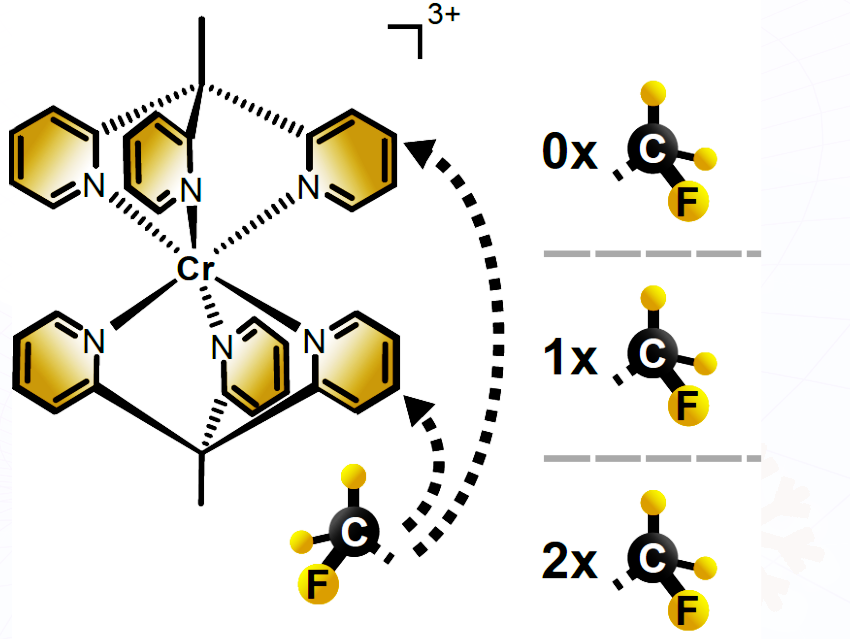
Katja Heinze, Robert Naumann, and Steven Sittel, Johannes Gutenberg University, Mainz, Germany, and their co-authors have synthesized a series of chromium(III) chromophores with identical optical properties but systematically tunable redox behavior to study photoredox mechanisms.
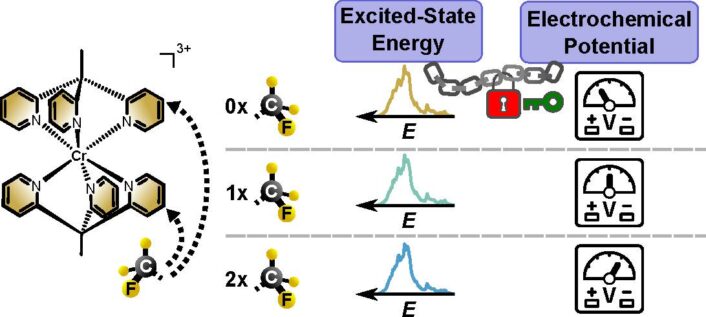
To do so, the researchers synthesized polypyridine chromium(III) complexes with tripodal ligands (tpe) modified by one or two trifluoromethyl (CF₃) groups. Introducing these electron-withdrawing substituents selectively increased the complexes’ redox potentials without affecting their spin-flip excited-state energies or lifetimes. In other words, the CF₃ groups altered the complexes’ redox properties without changing their photophysical characteristics.
What did you do?
We created a series of chromium complexes that differ in their readiness to accept electrons (reduction potential), a critical step in their role as photoredox catalysts. In contrast, their structures and interactions with light are virtually identical, including the fraction of light absorbed, the amount of light energy stored (excited-state energy), and the duration of energy storage (excited-state lifetime).
Why are you interested in this?
Research on the mechanistic aspects of photoredox catalysis is highly challenging, as every individual step of the catalytic cycle is influenced by numerous factors. Insight can be gained by comparing, e.g., different catalysts.
However, in basically all common photocatalysts, excited-state energies and reduction potentials are tightly linked and cannot be adjusted independently. Thus, switching between catalysts in mechanistic research studies changes multiple variables, making the influence of any single parameter unclear.
To better understand the elementary steps in photoredox catalysis, we aimed to disentangle these parameters.
What is new and cool about your research?
The cool part about this research is the highly unusual separation of variables in these complexes. While excited-state energies and reduction potentials are typically intertwined, this is not the case here. Thus, these compounds essentially differ merely in their ground and excited-state redox potentials but share optical properties and excited state lifetime.
They can, therefore, serve as a unique tool for deep mechanistic research. A detailed understanding of the underlying mechanisms is key to achieving targeted and systematic optimization of photocatalytic reactions.
What specific applications do you imagine?
We are excited to say that specific applications have already begun in our laboratories.
Currently, research on electron transfer processes is being conducted, comparing these new chromium(III) compounds. In doing so, we aim to assess the isolated influence of changes in the reduction potential and, consequently, the thermodynamic driving force. This research should provide deep insight and understanding into the elementary steps of light-driven catalysis.
What part of your work was the most challenging?
The biggest challenge was the synthetic access to these compounds, as both the metal and the ligands are electron-deficient, making bond formation less favorable.
The key was providing sufficient activation energy and driving force, using a microwave-assisted synthesis and a solvent mixture that that caused the desired product to precipitate as it formed.
Additionally, while the obtained products displayed good purity for conventional applications, they required purification by HPLC for spectroscopic analysis. Since translation from analytical to preparative column was not straightforward, the development of a suitable method proved challenging—but succeeded in the end. Now reliable optical data are available that will enable next steps in mechanistic research.
Thank you very much for sharing these insights.
The paper they talked about:
- Decoupling Redox Potentials and Excited State Energies in Substituted Chromium(III) Chromophores,
Steven Sittel, Dimitri Zorn, Alexandra König, Jonas Marcel Grenz, Christoph Förster, Robert Naumann, Katja Heinze,
Chemistry – A European Journal 2025.
https://doi.org/10.1002/chem.202502668
Katja Heinze, Robert Naumann, and Steven Sittel are researchers in the Department of Chemistry at Johannes Gutenberg University in Mainz, Germany.
Also of Interest