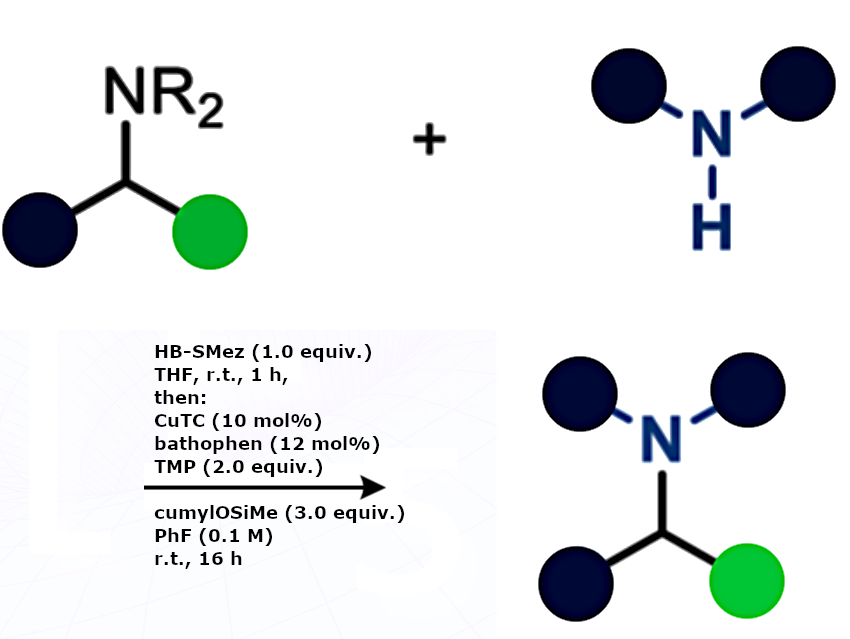
Amines are among the most common functional groups in bioactive molecules and pharmaceuticals. Although common, amines are usually treated as synthetic endpoints rather than reactive sites, because direct manipulation of the carbon–nitrogen bond has been chemically challenging.
Daniele Leonori, RWTH Aachen University, Germany, and colleagues have developed a strategy that repositions native primary, secondary, and tertiary amines as handles for cross-coupling. The platform relies on in situ activation via borane coordination and uses a copper catalytic redox system that generates amine-ligated boryl radicals, which undergo β-scission across the C(sp³)–N bond to release alkyl radicals. These intermediates undergo copper-catalyzed cross-couplings with a broad array of C-, N-, O-, and S-based nucleophiles.
The researchers were able to activate diverse classes of amines and achieve successful cross-couplings with a wide variety of other organic molecules, allowing the synthesis of numerous derivatives. Example of the general procedure for the transamination reaction shown above. Amides can also be converted into reactive intermediates and incorporated into the same reaction pathway via reductive funneling.
This method allows chemists to make small but crucial modifications to pharmaceutical molecules, making it easier to produce chemically diverse drugs with different biological functions—efforts that previously required many tedious steps. According to the researchers, this work establishes a general approach to deaminative C–N bond functionalization and represents a first step toward using amines as reactive handles in chemical synthesis.
- Deaminative cross-coupling of amines by boryl radical β-scission
Zhenhua Zhang, Giovanni Lonardi, Thomas Sephton, Yusuf C. Guersoy, Chiara Stavagna, Giovanni V. A. Lenardon, Massimo Bietti, Daniele Leonori
Nature 2025.
https://doi.org/10.1038/s41586-025-09725-1