Polycyclic aromatic hydrocarbons (PAHs) in organic crystals are key quantum emitters, producing high-purity, narrow-bandwidth photons ideal for single-photon applications. Their tunable properties make them benchmarks in organic quantum devices. One of the most advanced PAH-based single-photon emitters iis 7,8:15,16-dibenzoterrylene (DBT). When embedded in a crystal matrix, DBT shows high quantum efficiency and stable emission wavelengths.
DBT was synthesized by Erich Clar and Winfried Willicks in 1955 [1]. However, due to its limited solubility and stability in solution, no other documented attempts at its synthesis and comprehensive characterization have been reported to date. Alexander S. Oshchepkov, Konstantin Yu. Amsharov, Martin-Luther-University Halle-Wittenberg, Halle (Saale), Germany, and colleagues have performed the first comprehensive experimental and computational characterization of DBT, revealing its unusual biradical electronic behavior.
They combined 2D NMR spectroscopy, powder X-ray diffraction (PXRD), EPR measurements, and density functional theory (DFT) calculations to determine DBT’s structure, conformational landscape, and electronic properties. They examined how stereoisomer interconversion and solvent environment affect its biradical character. The goal was to understand and control DBT’s geometry-induced biradical behavior, providing a design principle for stable, low-gap open-shell organic molecules with potential applications in spintronics, quantum information science, and responsive organic materials.
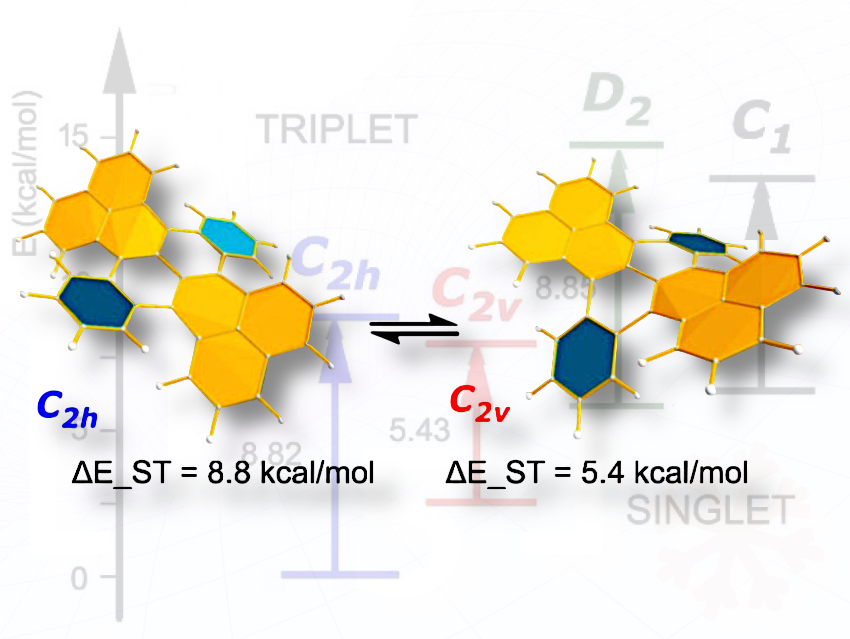
DBT can exist in four distinct conformers—C₂h, C₂v, D₂, and C₁—which are expected to be in thermal equilibrium at room temperature.
The researchers found that DBT is the first studied molecule with two low-lying conformers exhibiting drastically different biradical character. It shows a unique conformational switch, where one conformer is essentially closed-shell and the other has pronounced biradical character. Radical centers are localized at the molecular core rather than the periphery, contributing to stability despite inherent biradical reactivity. Its electronic properties are geometry-dependent: interconversion between stereoisomers dramatically changes the biradical character and the singlet–triplet energy gap, allowing access to tunable near-degenerate states. Furthermore, solvent affects the population of the biradical state.
A detailed DFT analysis revealed that the non-planar geometry of the molecule plays a key role in the emergence of biradical character. The findings suggest that DBT can be viewed as a Clar-sextet-interlocked biphenalenyl.
The ability to control electronic ground states via molecular geometry makes DBT a promising candidate for molecular spintronics, quantum devices, and responsive organic materials.
- The Mystery of Dibenzoterrylene: A Clar-interlocked Biphenalenyl Biradical
Elena A. Kalinina, Mikhail A. Kalinin, Dmitry I. Sharapa, Harald Maid, Alexandra Freidzon, Haleh Hashemi Haeri, Dariush Hinderberger, Robert Dennebier, Alexander S. Oshchepkov, Konstantin Yu. Amsharov
Chem. Eur. J. 2025.
https://doi.org/10.1002/chem.202502952
[1] Erich Clar, Winfried Willicks, Aromatische Kohlenwasserstoffe, LXIX. Mitteil.: 7.8;15.16-Dibenzterrylen, Chemische Berichte 1955, 88(8), 1205–1207. https://doi.org/10.1002/cber.19550880809