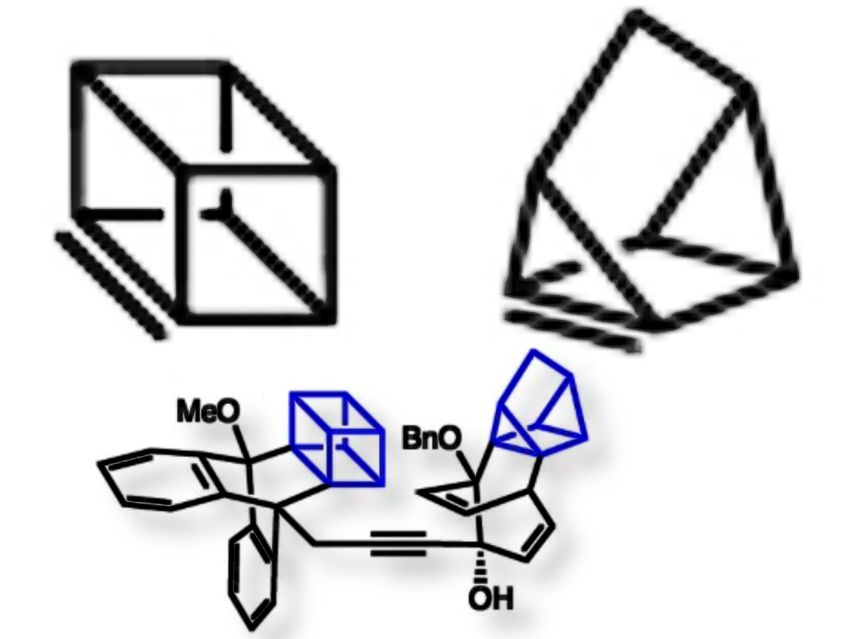
Neil K. Garg, University of California, Los Angeles, USA, and colleagues have used highly strained alkenes—cubene and 1,7-quadricyclene—to provide a straightforward way to build highly complex, three-dimensional molecules of interest to medicinal chemists.
Alkene carbons are typically trigonal planar, as in ethylene, allowing maximal p-orbital overlap. However, in confined ring systems, alkenes can deviate from this geometry. In hyperpyramidalized alkenes, the carbon geometry is significantly distorted, weakening the π-bond and lowering the bond order to around 1.5.
These unusually weak π-bonds make cubene and quadricyclene highly reactive in cycloaddition reactions, enabling the construction of rigid, three-dimensional frameworks with complex substitution patterns. This approach provides access to previously inaccessible molecular structures, valuable in medicinal chemistry, drug discovery, and materials science, and could inspire the design of other molecules with unusual bond geometries.
For example, cubene and quadricyclene can be combined into a single hybrid structure containing both cages, forming an intricate 3D molecule with four contiguous carbon centers, a rare and visually striking scaffold.
According to the researchers, this work paves the way for designing and using other unconventional intermediates with hyperpyramidalized or non-integer bond orders in chemical synthesis.
In addition, the researchers have developed mild synthetic methods to generate and trap cubene and 1,7-quadricyclene. Previously, experimental studies required harsh conditions, such as strong bases or reactive organolithium reagents, which limited the scope of reactions and practical applications. Cubene was generated from suitably functionalized cubane precursors (Kobayashi-type) and then trapped in Diels–Alder cycloadditions with reactive dienes. 7-Quadricyclene was produced via base-mediated dehydrohalogenation of precursors under milder conditions than the original 1979 method, followed by cycloaddition trapping to capture the reactive strained alkene.
- Hyperpyramidalized alkenes with bond orders near 1.5 as synthetic building blocks
Jiaming Ding, Sarah A. French, Christina A. Rivera, Arismel Tena Meza, Dominick C. Witkowski, K. N. Houk, Neil K. Garg
Nature Chemistry 2026
https://doi.org/10.1038/s41557-025-02055-9https://doi.org/10.1038/s41557-025-02055-9