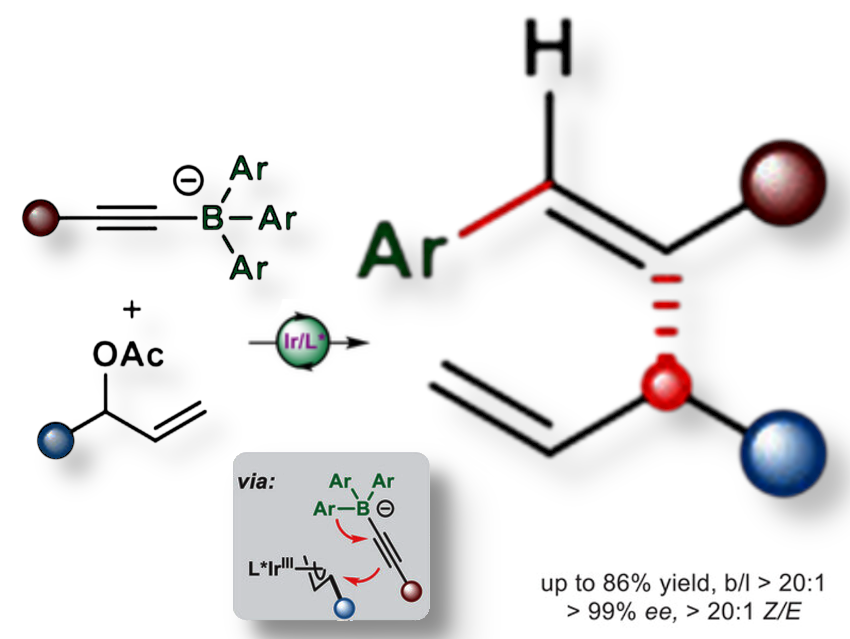
Skipped dienes (molecules with two double bonds separated by at least one single-bonded carbon), especially 1,4-dienes, are important building blocks in drug synthesis and complex molecule construction. However, selectively forming them from simple precursors remains challenging.
Lianrui Hu, East China Normal University, Shanghai, China, Liang Fu and Hui Wang, Anhui Normal University, Wuhu, China, and colleagues have developed an iridium-catalyzed enantioselective allylation of allylic electrophiles with alkynyl boronates producing 1,4-dienes with high Z/E selectivity and excellent enantioselectivity. The reaction enables the selective installation of a chiral allyl group onto target molecules.
The reaction process involves an allylation-induced 1,2-migration of the alkynyl boronate, followed by syn-addition of the migrating group and Ir(π-allyl) complex across the alkyne fragment, favoring formation of Z-alkenes. The iridium catalyst precisely controls the stereochemistry, yielding one enantiomer preferentially.
According to the researchers, this method accommodates a broad range of substrates, and the resulting enantiomerically enriched 1,4-dienes can be readily transformed into diverse derivatives, offering a versatile route for complex molecule synthesis.
- Iridium-Catalyzed Enantioselective Allylation of Alkynylboronates to Access Chiral 1,4-Dienes
Fengya He, Ziyi Sun, Xu Zhang, Zhen Long, Quansheng Zhao, Lianrui Hu, Liang Fu, Hui Wang
Angew. Chem. Int. Ed. 2026
https://doi.org/10.1002/anie.202523810