Benzofurans are an important class of chemical compounds, found in many natural products and biologically active molecules. Derivatives such as 3-unsubstituted 2-arylbenzofurans often have biological activities such as anticancer, antimicrobial, and antioxidant properties. However, methods for their synthesis are often complex, involve the use of expensive reagents or catalysts, or have low yields. One possible path is the sequential Michael addition and cyclization of 1,3-dicarbonyl compounds with 1,4-benzoquinones. However, ketones are rarely used in this reaction because of their low nucleophilicities.
Fengtian Wu, Rongxian Bai, and Yanlong Gu, Huazhong University of Science and Technology, Wuhan, China, have developed a synthesis of benzofurans from ketones and 1,4-benzoquinones that solves this problem. The team used triethyl orthoformate as an additive and Sc(OTf)3 as a catalyst. The additive forms a vinyl ethyl ether with the ketone, which enhances the nucleophilicity and allows the Michael addition.
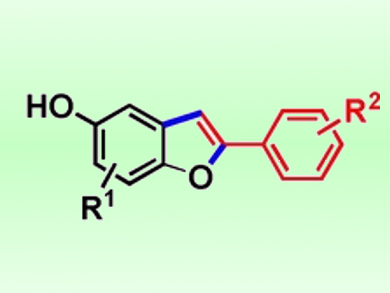
The researchers used this approach to synthesize a number of 5-hydroxy-2-arylbenzofurans (pictured) in good yields. Synthetic targets included an intermediate for the synthesis of tanshinone IIA (a phosphatase inhibitor), a precursor for an anticancer compound that can inhibit the mTOR enzyme, and 5-cyano-2-arylbenzofuran, a liquid crystal component. According to the team, the method can also be used to produce 2-phenylbenzofuran derivatives. The hydroxyl group in the products can be used as a reactive site to introduce other functional groups.
- Synthesis of Benzofurans from Ketones and 1,4-Benzoquinones,
Fengtian Wu, Rongxian Bai, Yanlong Gu,
Adv. Synth. Catal. 2016.
DOI: 10.1002/adsc.201600048