Over 55 % of the total rubber production comes from synthetic rubber—15 million tons of which are produced every year. Commonly used catalysts for this process are multicomponent Ziegler-type catalysts that contain rare-earth metals. On the one hand, these catalysts give high cis-1,4 selectivity in the polymerization of 1,3-dienes, but on the other the products have a broad range of molecular weights and molecular-weight distributions.
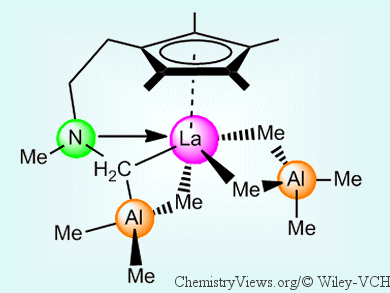
With the aim of finding out more about the factors that affect the stereoselectivity and control of molecular-weight distributions, Reiner Anwander and his group, University of Tübingen, Germany, have developed a series of new pre-catalysts. These are based on half-sandwich rare-earth-metal bis(tetramethylaluminate) complexes that contain amino-functionalized tetramethylcyclopentadienyl ligands. Starting from [Ln(AlMe4)3] (Ln = La, Nd, Y) complexes and the functionalized cyclopentadienes, the half-sandwich complexes were easily obtained by means of a protonolysis procedure. Further selective C–H bond activation of an aminomethyl group on the ligand led to a (μ-CH2) linkage between the AlMe3 moiety, the rare-earth metal center, and the amino nitrogen atom (pictured).
These linked half-sandwich complexes displayed excellent activity (up to 95.6 trans-1,4 selectivity) in the polymerization of isoprene upon the addition of fluorinated organoboron reagents as co-catalysts. In addition, very narrow molecular-weight distributions were achieved upon activation with [PhNMe2H][B(C6F5)4] as a co-catalyst in toluene.
- Rare-Earth-Metal Alkylaluminates Supported by N-Donor-Functionalized Cyclopentadienyl Ligands: C–H Activation and Performance in Isoprene Polymerization,
L. N. Jende, C. Maichle-Mössmer, R. Anwander,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302388