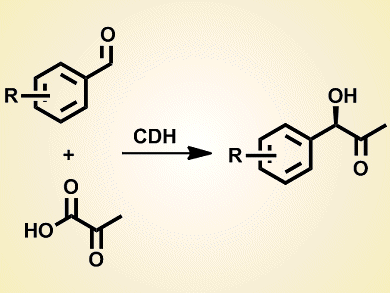
Cyclohexane-1,2-dione hydrolase (CDH) is a thiamine diphosphate (ThDP)-dependent enzyme that catalyzes the C–C bond-forming cross-benzoin reaction of benzaldehydes with pyruvate.
Michael Müller and co-workers, Albert-Ludwigs-Universität, Freiburg, Germany, determined the range of substrates accepted by recombinant CDH. Instead of isolating CDH from its native source (Azoarcus sp. strain 22Lin) they used a synthetic gene construct for optimal production of the enzyme.
Twenty-four monosubstituted benzaldehydes were tested in the reaction with pyruvate. Hydroxybenzaldehydes, nitrobenzaldehydes, and naphthaldehydes are also suitable substrates for CDH, unlike for other ThDP-dependent enzymes. Most importantly, as 20–30 % DMSO can be tolerated in the reaction media, hydrophobic and sterically demanding aromatic aldehydes are also appropriate substrates.
The straightforward production of CDH combined with its stability and multifunctional catalytic activity make it an interesting biosynthetic catalyst for many applications.
- Catalytic Scope of the Thiamine-Dependent Multifunctional Enzyme Cyclohexane-1,2-Dione Hydrolase,
S. Loschonsky, S. Waltzer, S. Fraas, T. Wacker, S. L. A. Andrade, P. M. H. Kroneck, M. Müller,
ChemBioChem 2014.
DOI: 10.1002/cbic.201300673