The Suzuki coupling reaction is an efficient tool for the construction of C–C bonds. However, it usually requires expensive Pd-based catalysts. The development of ligand-free and, preferably, Pd-free catalytic systems for Suzuki reactions is, thus, an interesting research target.
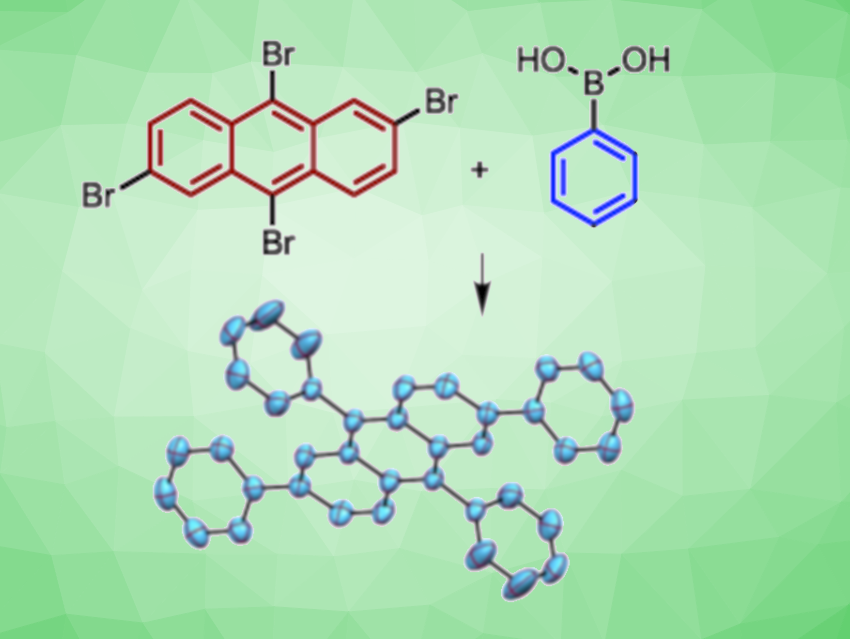
Akshai Kumar, Indian Institute of Technology Guwahati, Assam, India, and colleagues have found that inexpensive, earth-abundant nickel bromide can be used to catalyze the Suzuki coupling of a variety of aryl halides with different arylboronic acids (example pictured). In addition to NiBr2 as the catalyst, the team used K3PO4 as a base and 1,4-dioxane as the solvent. The reactions were performed at 120 °C.
The reaction shows good functional group tolerance and provides high yields under ambient, ligand-free, and activator-less conditions. The reactivity of the aryl halides follows the order ArI > ArBr > ArCl, in accordance with the bond strengths between the carbon and halogen atoms.
Using this approach, valuable new polycyclic aromatic hydrocarbons with interesting photophysical properties have been synthesized via a nickel(II) catalyzed multi-fold Suzuki coupling of tetrabromoanthracene and tetraiodobenzene with arylboronic acids. The team proposes a nickel(0)/nickel(II) catalytic cycle, where an oxidative addition of the aryl halide to nickel(0) is likely to be the rate-determining-step.
- Nickel Bromide Catalyzed Ligand‐Free and Activator‐less Suzuki Coupling Reactions,
Khadimul Islam, Vinay Arora, Vikas Vikas, Bedabara Nag, Akshai Kumar,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200440



Very Nice