Polypyridyl cobalt complexes are noble-metal-free, stable catalysts for the reductive half-reaction of water splitting, i.e., the H2 evolution reaction (HER). This reaction could be important for storing sustainable energy. Solar energy, for example, could be stored in the form of hydrogen rather than in expensive batteries. However, high overpotentials generally hamper the application of such cobalt complexes.
Roger Alberto, University of Zurich, Switzerland, and colleagues have synthesized two dinuclear Co-polypyridyl catalysts (pictured below) for the HER. In these complexes, two cobalt cores are bridged by bipyridine or pyrazine units. The team first prepared the ligands via double lithiation of 6,6′-dibromobipyridine or 2,5-dibromopyrazine, respectively, followed by a nucleophilic addition to di-(2,2′-bipyridin-6-yl)-methanone. The complexes were then synthesized by adding excess Co(BF4)2 to the respective ligand.
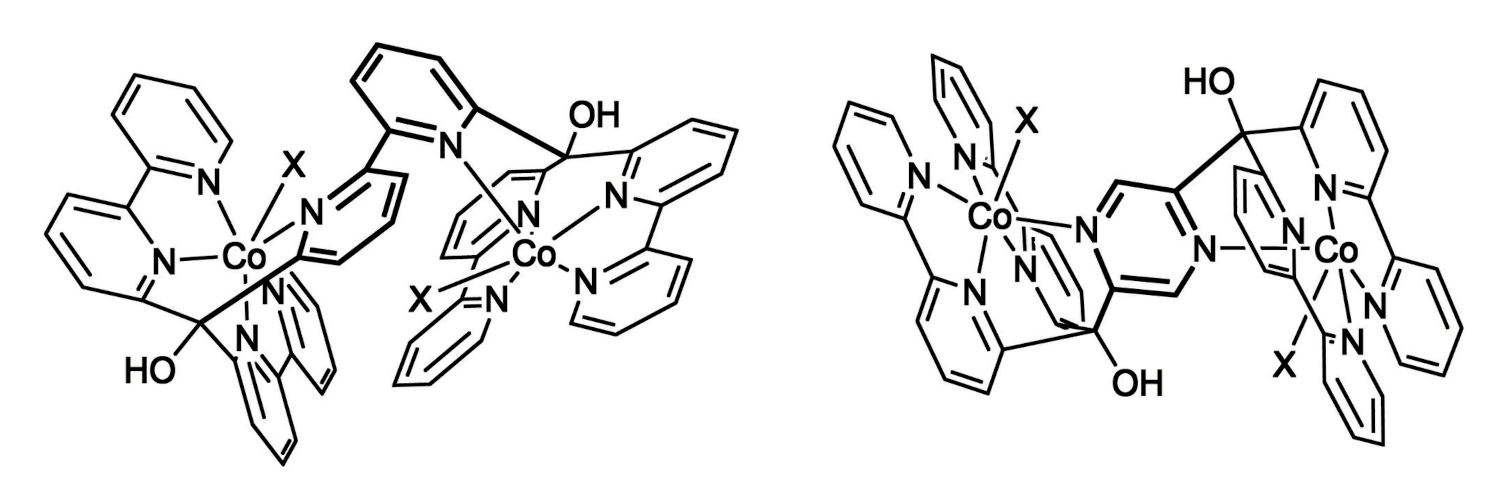
The team observed turnover numbers (TONs) for photocatalytic HER of up to 20,000 for the pyrazine-linked complex (pictured on the right, X = MeCN) and 7,000 for the bipyridine-linked (pictured on the left, X = μ-F) complex. The pyrazine-linked complex showed electronic coupling between the two Co centers. This interaction allows for easier protonation of the complex and improves the efficiency of H2 production. The complex with cooperating cobalt centers outperformed both its mononuclear analogue and the dicobalt complex without electronic interaction.
The work demonstrates the cooperativity of the cobalt centers in a dual-core catalyst featuring electronically coupled cobalt centers.
- Two Novel Dinuclear Cobalt Polypyridyl Complexes in Electro‐ and Photocatalysis for Hydrogen Production: Cooperativity increases Performance,
Nicola Weder, Nora S. Grundmann, Benjamin Probst, Olivier Blacque, Rangsiman Ketkaew, Fabrizio Creazzo, Sandra Luber, Roger Alberto,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202201049