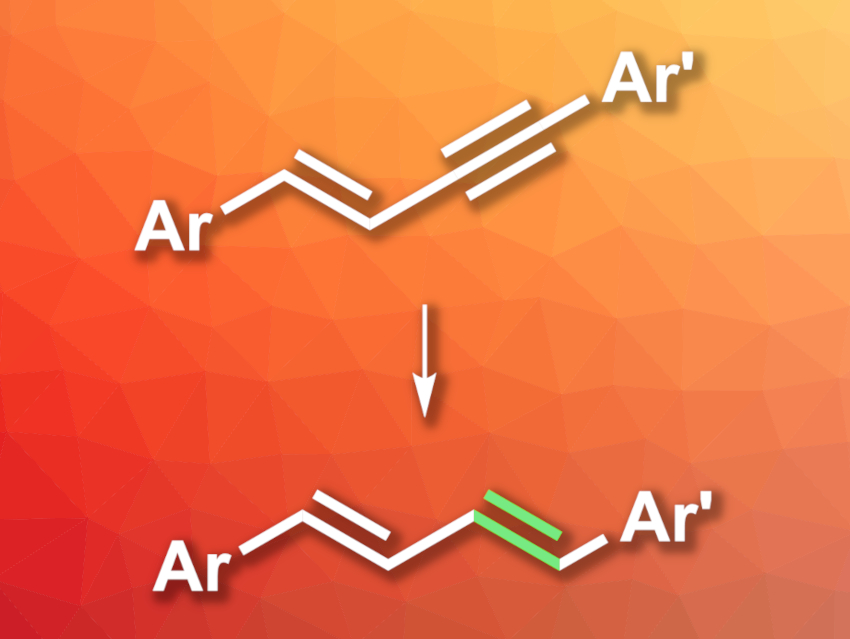
Conjugated alkenes are useful, e.g., in organic synthesis, materials, or bioactive compounds. Many methods for their synthesis suffer from drawbacks such as poor atom economy due to the formation of byproducts. The semihydrogenation of 1,3-enynes could provide an alternative path to conjugated dienes with high atom economy. However, this type of reaction can have selectivity issues due to overreduction or stereoselectivity concerns.
Guixia Liu, Shanghai Institute of Organic Chemistry and Hangzhou Institute of Advanced Study, both University of the Chinese Academy of Sciences, and colleagues have developed a selective trans-semihydrogenation of 1,3-enynes (general reaction pictured) that provides access to (E,E)-butadienes. The team reacted different 1,3-enynes with ethanol, which serves as both a hydrogen source and a solvent, in the presence of t-BuNH2, using an iridium(III) hydrido chloride pincer complex with bulky adamantyl substituents as the catalyst.
The desired dienes were obtained in mostly good to excellent yields and high stereoselectivities. The reaction shows good functional group tolerance, including, e.g., reducible ester groups. The team proposes a reaction mechanism that involves a cis-semihydrogenation of the alkyne group to give an (E,Z)-diene at first, followed by (E,Z)-to-(E,E) isomerization.
- Iridium-Catalyzed Selective trans-Semihydrogenation of 1,3-Enynes with Ethanol: Access to (E,E)-1,4-Diarylbutadienes,
Fengjie Huang, Zhidao Huang, Guixia Liu, Zheng Huang,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02327