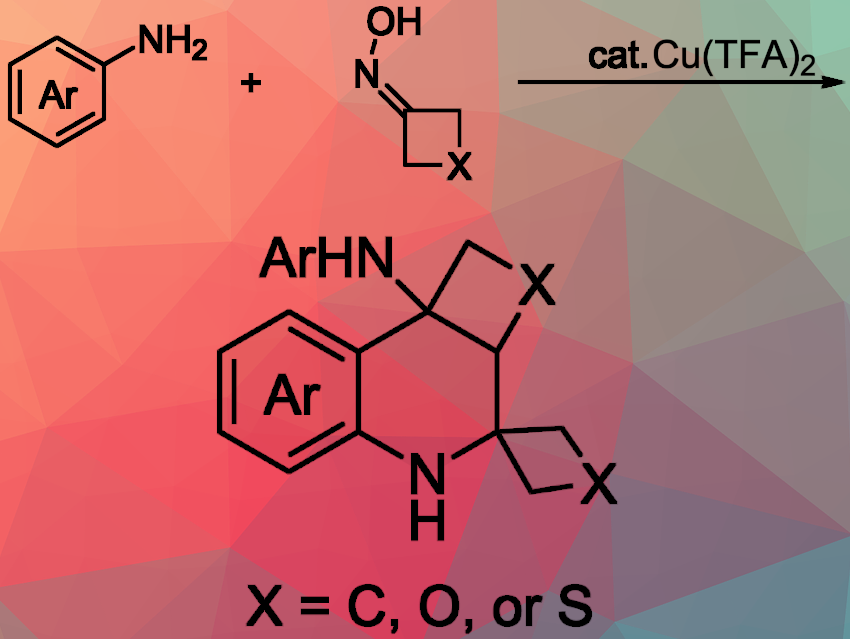
Tetrahydroquinolines (THQs) are important scaffolds in medicinal chemistry. Cyclobutane-fused THQs have attracted attention in drug discovery due to their inherent structural rigidity and enhanced pharmacological profiles. The strained cyclobutane ring, in particular, acts as a versatile and conformationally constrained building block, enabling the construction of complex molecular architectures with potent biological activities. The strained cyclobutane ring helps build complex molecules with strong biological activity. However, current methods for making fused THQs often involve multistep preparation and complicated procedures, limiting their practical use and scalability.
Pan Gao, Yu Yuan, and colleagues, Yangzhou University, China, have developed an efficient and convenient method for synthesizing cyclobutane-fused and conformationally constrained spirotetrahydroquinolines (STHQs) from arylamines and cyclobutanone oxime using a copper-catalyzed reaction under ambient air conditions. The team optimized the reaction conditions to effectively form tetrahydroquinoline derivatives with varying substituents, achieving high yields under mild conditions. Hexane wasused as the solvent, and copper(II) trifluoroacetate (Cu(TFA)₂) as the catalyst (20 mol %) under ambient air at 80 °C for 12 hours.
Mechanistic studies suggest the following catalytic cycle: In the presence of a copper catalyst, aniline reacts with cyclobutanone oxime to form an imine intermediate, which undergoes isomerization to generate an enamine intermediate. Subsequently, an intermolecular cyclization occurs between the enamine and imine intermediates, ultimately yielding the final product through an aromatization process.
This method provides a selective approach for generating bioactive tetrahydroquinoline scaffolds, which have broad applications in pharmaceutical chemistry. The team demonstrated the scalability of this method through a gram-scale reaction, highlighting its potential for practical applications in medicinal chemistry and drug discovery.
- Copper-catalyzed domino cyclization of anilines and cyclobutanone oxime: a scalable and versatile route to spirotetrahydroquinoline derivatives,
Qingqing Jiang, Xinyi Lei, Pan Gao, Yu Yuan,
Beilstein J. Org. Chem. 2025, 21, 749–754.
https://doi.org/10.3762/bjoc.21.58