Transition metals (TMs) typically show rich redox chemistry and can be found in various oxidation states. While strong π-accepting ligands have stabilized molecular TM complexes with TMs in formal negative oxidation states, organic-ligand-free TM anions remain rare and are largely restricted to intermetallic compounds based on heavy TMs like gold or platinum.
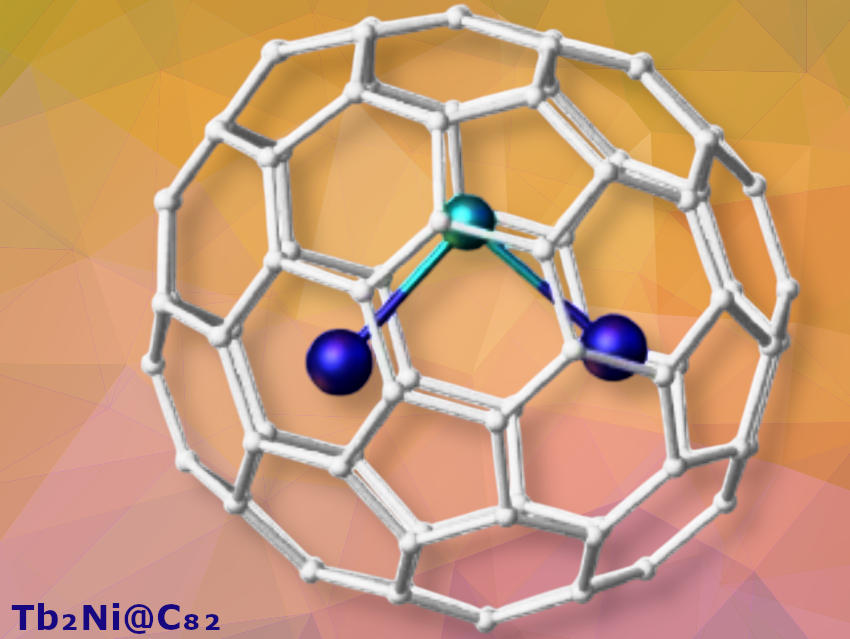
Ziqi Hu (Peking University, Beijing, China, and University of Science and Technology of China, Hefei), Eugenio Coronado (Universidad de Valencia, Paterna, Spain), Shangfeng Yang (University of Science and Technology of China), Zujin Shi (Peking University), and colleagues have reported the bulk synthesis and characterization of an air-stable [(Tb³⁺)₂Ni²⁻]⁴⁺ cluster encapsulated in fullerenes (Tb₂Ni@C₈₂), marking a significant advance in stabilizing ligand-free nickelide anions. This charged lanthanide–nickelide cluster forms metal-only Lewis pairs with highly polarized Tb–Ni covalent bonds and remarkably short bond lengths (2.50–2.57 Å) This is consistent with strong back-donation from the electron-rich Ni²⁻ center (3d¹⁰4s² configuration) to empty 5d orbitals on Tb³⁺.
These results show the pseudo-sulfide character of the Ni²⁻ anion and solve a long-standing question of whether iron-group elements can be captured by fullerenes. This work demonstrates that intermetallic clusters with ligand-free TM anions can be stabilized within a confined carbon sub-nanospace. According to the team, the approach could be extended to other derivatives such as Y₂Ni clusters.
- Lanthanide–nickel molecular intermetallic complexes featuring a ligand-free Ni2− anion in endohedral fullerenes,
Panfeng Chuai, Ziqi Hu, Yang-Rong Yao, Zhanxin Jiang, Aman Ullah, Ya Zhao, Weiren Cheng, Muqing Chen, Eugenio Coronado, Shangfeng Yang, Zujin Shi,
Nature Chemistry 2025.
https://doi.org/10.1038/s41557-025-01802-2