Hydrodehalogenation on an sp2 carbon is an important organic transformation in synthesis. It is industrially applied for the detoxification of hazardous environmental chemicals as well as for deprotection strategies in synthetic chemistry. This approach also allows for the incorporation of deuterium into medicinal compounds. To date, a number of methods have been developed, many of which may give rise to potential environmental or health risks.
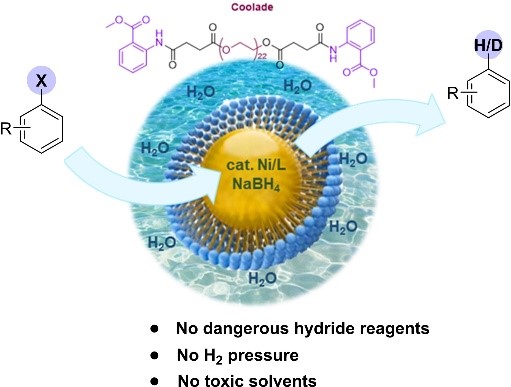
Lipshutz and co-workers report a nickel-catalyzed direct and environmentally friendly protocol for hydrodehalogenation and dehalogenative deuteration of (hetero)aryl halides using water as the reaction medium. The procedure can be applied to a diverse range of halogen-containing substrates, such as aromatic bromides and chlorides, and selected heteroaromatic fluorides. The utility of the method was also illustrated by the synthesis of the drug rac-bitopertin, which is currently used for the treatment of schizophrenia.
By employing recyclable water rather than organic solvents, this approach offers significant environmental benefits. The relatively low E-Factors further demonstrate the cost-effectiveness and sustainability of such reductions.
Ref:
- Nickel-Catalyzed Hydro- and Deutero-dehalogenations of (Hetero)Aryl Halides under Aqueous Micellar Catalysis Conditions
Monica S. Lopez Lemus, Dr. Rahul D. Kavthe, Rohan M. Thomas, Max Baumann, Dr. Karthik S. Iyer, Bruce H. Lipshutz
ChemSusChem 2025
https://doi.org/10.1002/cssc.202500043