Huan-Ming Huang, ShanghaiTech University, Shanghai, China, and his co-authors use blue light and palladium to transform flat molecules into rigid 3D cages, opening doors for next-generation drugs.
What did you do?
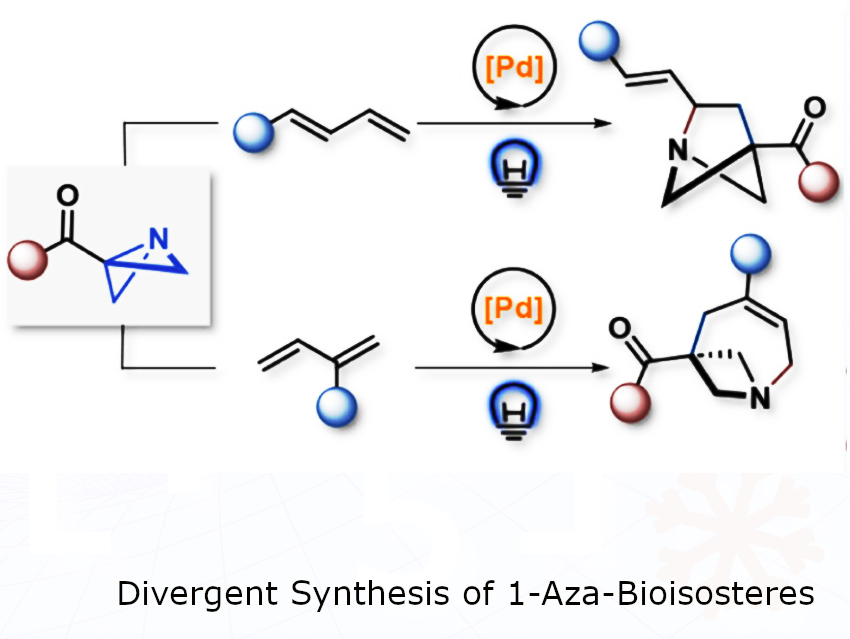
We created an efficient and versatile method to build rigid, three-dimensional (3D) carbon-nitrogen cage structures that can be used to enhance the properties of potential drug molecules.
Using the metal palladium and blue light, we act like molecular architects, taking common flat ring structures and “stitching” them into new, complex 3D shapes in a single step. This approach uses light and a palladium catalyst to transform a flat molecule into a 3D cage.
What are your key findings?
We developed a general and “divergent” tool. From one common starting material (azabicyclo[1.1.0]butanes), we can selectively build many different sizes of these valuable 3D cages simply by changing one reaction component—the ligand on the metal. This provides a practical and rapid way for drug discoverers to access a whole new library of 3D structures for testing.
Why are you interested in this research?
Many drug molecules are relatively flat, but the proteins they target in the body are complex 3D structures. Replacing a flat part of a drug with a 3D cage of similar size (a “bioisostere”) can make the drug fit its target better, improving its effectiveness and reducing side effects.
However, these specific cages are notoriously difficult to build. We set out to solve that problem.
So what is new and cool about your work?
The cool part is the fusion of light energy with palladium catalysis. Light provides the clean burst of energy needed to force the palladium catalyst to perform the difficult task of closing two chemical bonds at once to form the cage. This synergy creates a reaction that is both powerful and selective, something neither light nor palladium could do efficiently alone.
What part of your work was the most challenging?
The biggest challenge was achieving divergent control. We wanted one catalytic system to build different-sized cages on command. The key was finding the perfect “helper” molecules or ligands, for the palladium catalyst.
This involved testing dozens of ligands in a painstaking, trial-and-error process to see which one would guide the reaction. It was like finding the perfect key for multiple different locks, but without knowing what the keys should look like beforehand.
What specific applications do you imagine?
The most direct application is in pharmaceutical lead optimization. A chemist can take a promising drug candidate, use our method to replace a flat amine group with one of our 3D cages, and quickly see if it becomes a more potent, selective, and stable medicine.
The long-term vision is to make these 3D scaffolds readily available to improve the drug discovery process.
What comes next?
We are excited to see how the medicinal chemistry community will use these new scaffolds. We believe this work highlights the power of fundamental methodological research in organic chemistry to directly enable advances in other fields like biology and medicine.
And we are already exploring how to use this photochemical strategy to build other types of challenging 3D structures.
All the best for your research and thank you very much for sharing these insights.
The paper they talked about:
- Divergent Synthesis of 1-Azabicyclo[n.1.1]alkane Bioisosteres via Photoinduced Palladium Catalysis,
Ying Zhang, Kai-Dian Li, Song Yu, Kangyin Pan, Hongtao Xu, Huan-Ming Huang,
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.5c09500

Huan-Ming Huang is an Assistant Professor (Tenure Track) and Principal Investigator at ShanghaiTech University, Shanghai, China.
.
Also of Interest