Unusual bonding situations, such as 2-center-3-electron (2c3e) bonds, are interesting objects of chemical research. A simple example for a 2c3e bond would be the dihelium cation, in which two electrons occupy a bonding σ orbital and one electron occupies an antibonding σ* orbital. There are also some examples for 2c3e π-bonds, with a filled π orbital and a half-filled π* orbital. Generally, such a 2c3e π-bond has an underlying σ bond. However, single π bonds between pairs of atoms with no underlying σ bond exist, which suggested that 2c3e π bonds with no underlying σ bond are also accessible.
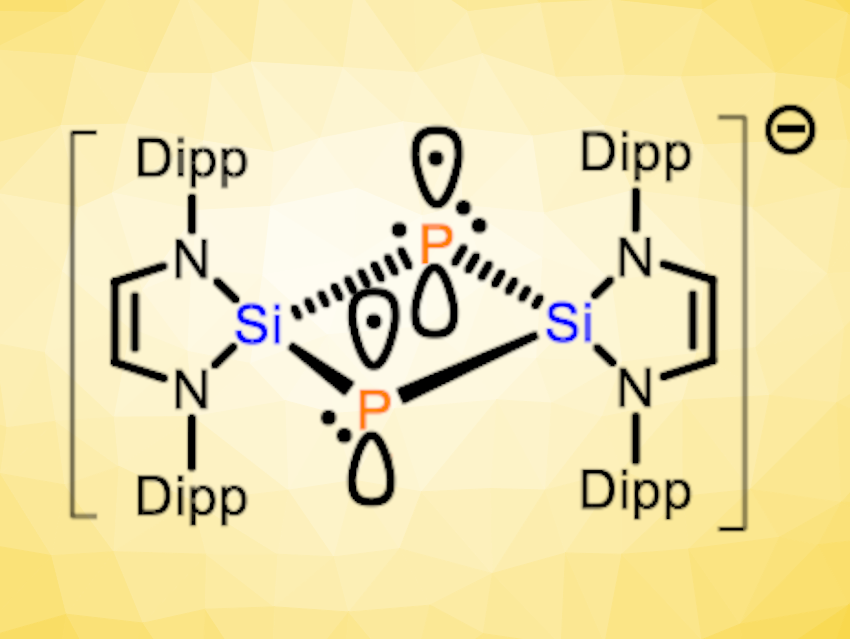
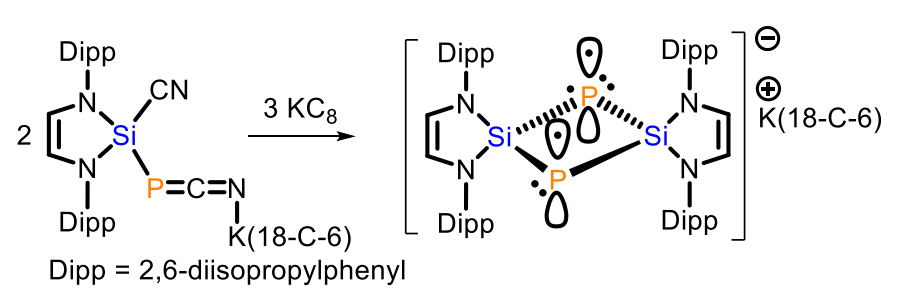
Liu Leo Liu, Southern University of Science and Technology, Shenzhen, China, and Nankai University, Tianjin, China, and colleagues have synthesized a compound with such a rare 2c3e π bond between two phophorus atoms, which exists in the absence of a supporting P–P σ bond (pictured above). The compound is a bicyclo[1.1.0]diphosphadisilabutane radical anion salt, namely, [K(18-C-6)]+[(HCNDipp)2Si]2P2}•− (18-C-6 = 18-crown-6, Dipp = 2,6-diisopropylphenyl). It was prepared starting from the N-heterocyclic silylene (HCNDipp)2Si, which was reacted with [K(18-C-6)][P(CN)2] to obtain (HCNDipp)2Si(CN)[PCNK(18-C-6)] (pictured below on the left). This intermediate was treated with KC8 to give the desired product.
According to the researchers, the compound represents the first example of a Si- and P-containing analog of a bicyclo[1.1.0]butane radical anion. X-ray diffraction showed that the radical anion features a planar four-membered Si2P2 ring, with Si‒P bond lengths that are close to those of Si‒P single bonds. The Si‒Si and P‒P distances are much longer than for the corresponding single bonds, but shorter than the sum of the van-der-Waals radii. In addition to X-ray diffraction crystallography, the team used electron paramagnetic resonance spectroscopy (EPR) and density functional theory (DFT) calculations to study this unusual compound. Overall, the results indicate that the species features a rare P‒P 2c3e π bond in the absence of a formal underlying P‒P σ bond.
- An Isolable Radical Anion Featuring a 2‐Center‐3‐Electron π‐Bond without a Clearly Defined σ‐Bond,
Yanbo Mei, Xiaodan Chen, Rui Wei, Xiao-Yong Chang, Lizhi Tao, Liu Leo Liu,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202315555