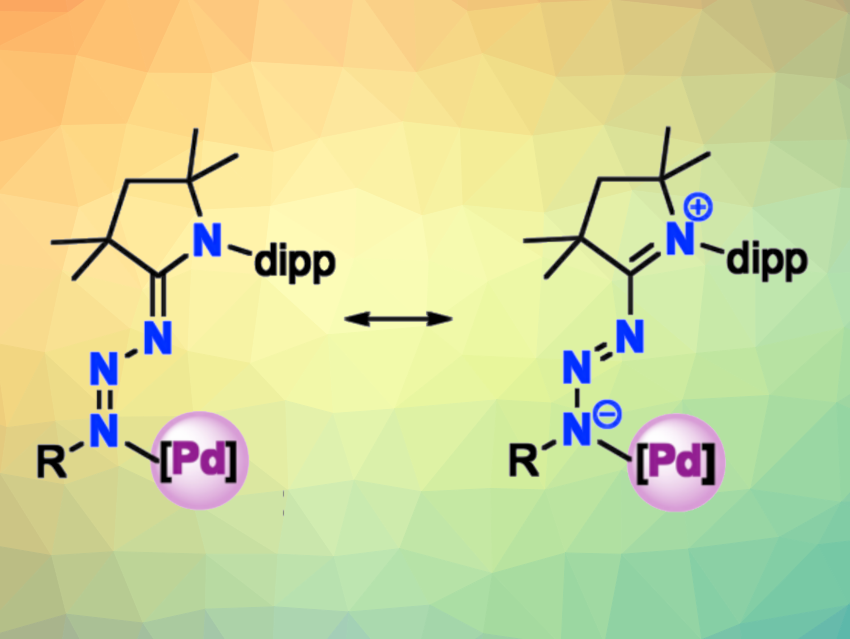
Nitrogen donor ligands are used in many important homogeneous transition-metal-catalyzed processes. Both neutral N-donor ligands, such as amines, and anionic N-donor ligands, such as amides, are used in this context. In addition to these ligand types with defined charges, there also are donor-flexible ligands, such as N-heterocyclic imines, which can dynamically switch between an anionic and a neutral character. Increasing the distance between the formal charges in such ligands could increase their donor ability.
Martin Albrecht, University of Bern, Switzerland, and colleagues have combined cyclic (alkyl)(amino)carbenes (CAACs) with a triazene to obtain donor-flexible ligands with an extended formal charge separation (example complex structures pictured). These ligands have a larger π-conjugted system that extends from the carbene through three nitrogens rather than just one as in N-heterocyclic imines. The team prepared three CAAC-triazene compounds with either a phenyl, dipp, or quinoline substituent R (dipp = 2,6-diisopropylphenyl) from the corresponding aryl azides and an N-dipp-substituted CAAC.
The researchers found that the new ligands are remarkably stable up to 120 °C without loss of N2. They also observed that a partially reversible E-to-Z isomerization within the triazene can be initiated by UV light, which further increases the flexibility of the ligands. The quinoline-substituted CAAC-triazene can serve as a chelating ligand for palladium. The resulting complex can serve as a precatalyst, e.g., for the oligomerization of ethylene or the isomerization of 1-hexene.
- Stable CAAC‐triazenes: A New Nitrogen Ligand System with Donor and Conformational Flexibility, and with Application in Olefin Activation Catalysis,
James J Race, Luke A Hudson, Martin Albrecht,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202400400