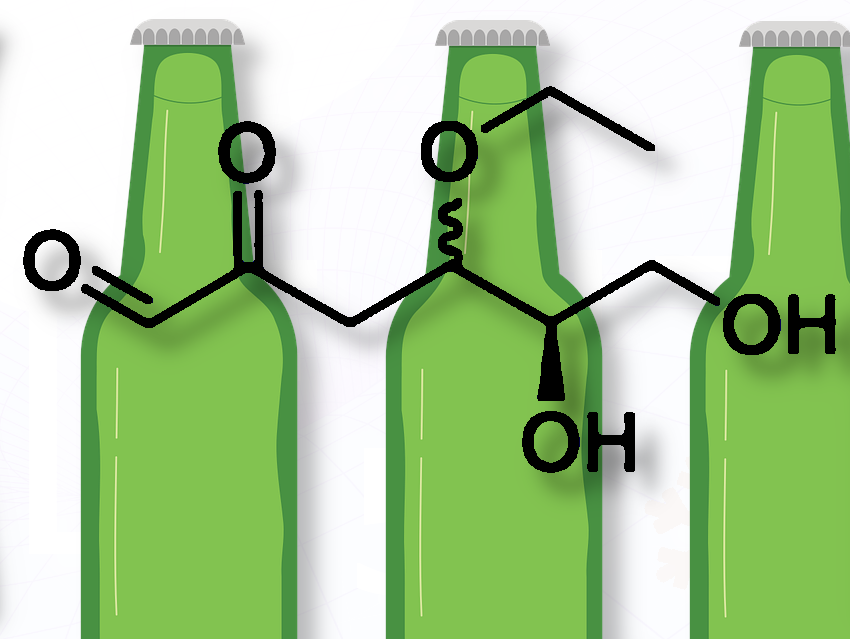
Michael Hellwig, TUD Dresden University of Technology, Germany, discusses his recent study showing that ethanol reacts with sugar-derived compounds in beer to form a new ethoxy dicarbonyl, revealing a non-enzymatic pathway that may contribute to beer aging and flavor changes.
What did you do?
The study was based on the hypothesis that certain unsaturated carbohydrates in beer, namely 3,4-dideoxyhexosone-3-enes, can react with ethanol to form etherified monosaccharides.
First, a model experiment was carried out to show that the reaction is possible. Next, the target compound was isolated in a derivatized form. Finally, the new compound was quantified across a broad range of beer samples.
Why are you interested in this?
During brewing and storage, sugars like glucose undergo caramelization (breakdown without amino acids) and glycation (reaction with amino acids, a Maillard-type reaction) to form 1,2-dicarbonyls such as 3-deoxyglucosone and 3-deoxygalactosone. These compounds can then react with ethanol in beer to form the newly discovered ethoxy dicarbonyls, showing a non-enzymatic pathway that may contributes to flavor development and aging.
Research on caramelization and glycation very often focuses only on the stability of the “starting materials”—for example, proteinogenic amino acids or monosaccharides—and often uses highly simplified model systems.
However, it is important to consider these structures and reactions in the context of the vast diversity of compounds present in a more realistic, complex food environment. That is why this work focused on the stability of an individual dicarbonyl compound in beer, which contains a substantial amount of the reactive compound ethanol.
What is new and cool about your study?
What is new and exciting about this work is that it provides the first evidence that ethanol is a reaction partner of unsaturated carbohydrates in beer. This expands the landscape of known sugar degradation pathways in brewing.
It is completely open if and how the new compounds might influence flavor development, aging characteristics, or even the chemical stability of beer.
What is the main significance of your results?
This work opens a new perspective on the chemical reactions of ethanol in food. While etherification of benzylic hydroxyl groups and esterification of organic acids have been described, this work highlights the addition of ethanol to a double bond.
Moreover, it shows that glycation reactions, i.e., non-enzymatic reactions of sugars, are not restricted to lysine or arginine residues, which is the classic pathway in glycation, but can also target hydroxyl groups present on other molecules, such as ethanol.
What specific applications do you imagine?
The concentrations of this compound in beer are significantly higher than those of other ethanol-derived compounds (e.g., furfuryl ethyl ether, ethyl esters). Thus, the 3-deoxy-4-ethoxyhexosones could represent compounds that are more sensitive as ageing markers in beer, because they can solely be formed after fermentation. Storage experiments together with sensory experiments are planned.
As the compound is ingested with beer, it will also be interesting to assess the bioactivity of the compound. A similar compound, 3-O-methylglucose, can upregulate the glucose transporter GLUT4. GLUT4 is an insulin-responsive membrane protein that mediates glucose uptake in muscle and fat cells.
What part of your work was the most challenging?
The most challenging aspect of this project was the preparation of the analytical standard. To date, the ethoxylated dicarbonyls—the actual compounds present in beer—could not be isolated. Only their derivatized form. The corresponding quinoxalines used for quantification could be obtained, as these derivatives are significantly more stable.
There is a lot of “hidden chemistry” in foods and beverages. Even after decades of research on beer, new compounds and pathways continue to emerge when we look closely. Understanding them not only enriches basic science but also may support improvements in product quality, consumer experience, and analytical practice.
Thank you very much for sharing these insights.
The paper they talked about:
- Formation of 3-Deoxy-4-ethoxyhexosones in Model Systems and Beer,
Michael Hellwig,
ChemFoodChem 2025.
https://doi.org/10.1002/cfch.202500038

Michael Hellwig is Chair of Special Food Chemistry at Technische Universität Dresden, Germany.
Current research topics include the chemistry of protein glycation and oxidation, as well as the study of vitamins and feed products.
Also of Interest