Mark Geoghegan, Newcastle University, Newcastle Upon Tyne, UK, and colleagues have developed a reversible, water-based glue that has good adhesion in the neutral pH range, but can be detached again in strongly acidic or alkaline environments. The new adhesive system, which is based on electrostatic interactions, has bond strengths somewhere between those of structural adhesives and pressure-sensitive adhesives. The adhesive also bonds “difficult”, low-energy surfaces such as polypropylene.
Types of Adhesives
Structural adhesive systems, such as two-component adhesives, form chemical bonds when they react, provide high tensile strength, and cannot be separated again. Pressure-sensitive adhesives, such as in adhesive tape or sticky notes, on the other hand, use physical bonding to provide weaker adhesion and can be removed by pulling.
The team found an alternative route to achieving adhesion and separation. Oppositely charged polyelectrolytes form electrostatic bonds along the interface. These charged species can be neutralized, and thus, the bond can be dissolved.
Complementary Water-Based Formulations
To create the desired charge interaction, the team developed two separate water-based polymer dispersions to be applied to the two surfaces that will be connected. In both dispersions, the base polymer is a copolymer composed of the inexpensive, commercially available components styrene and butyl acrylate.
Emulsion polymerization produces polymer nano- or microparticles formed of two phases, a hydrophobic core and a surfactant shell. Styrene and butyl acrylate were copolymerized through a free radical process to produce anionic and cationic emulsions depending on the material acting as surfactant.
The anionic emulsions were produced using sodium dodecyl sulfate (SDS) as an initial stabilizer and polymerizing acrylic acid as a shell in a second reaction. This provides a negative charge in the neutral to alkaline pH range. The cationic emulsions were produced using chitosan as the surfactant, providing positively charged amino groups in a neutral or acidic environment.
Good Adhesion
Both polymer dispersions formed sticky coatings on a variety of surfaces. The researchers observed that, when brought into contact, the coated surfaces stuck tightly together due to the electrostatic interactions between the positive and negative charges within the films.
This was even true in humid or wet environments, which usually have a detrimental effect on water-based adhesives. The electrostatic adhesive shows good adhesion even to low-energy surfaces such as polypropylene
A Reversible Connection
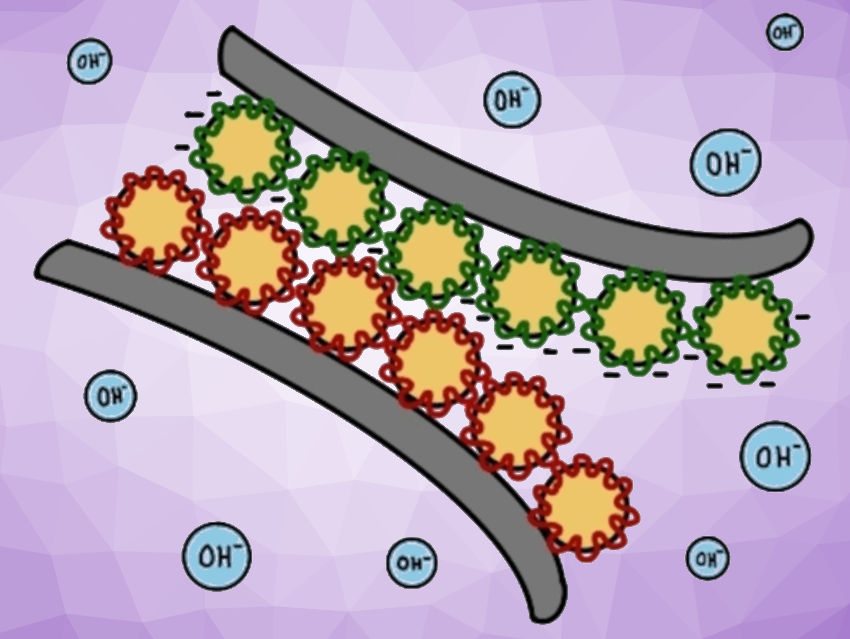
When the pH is adjusted to one extreme or the other, by adding either a strong acid or base, the negative or positive charges within the glue are neutralized, and the adhesion disappears (schematically pictured below).
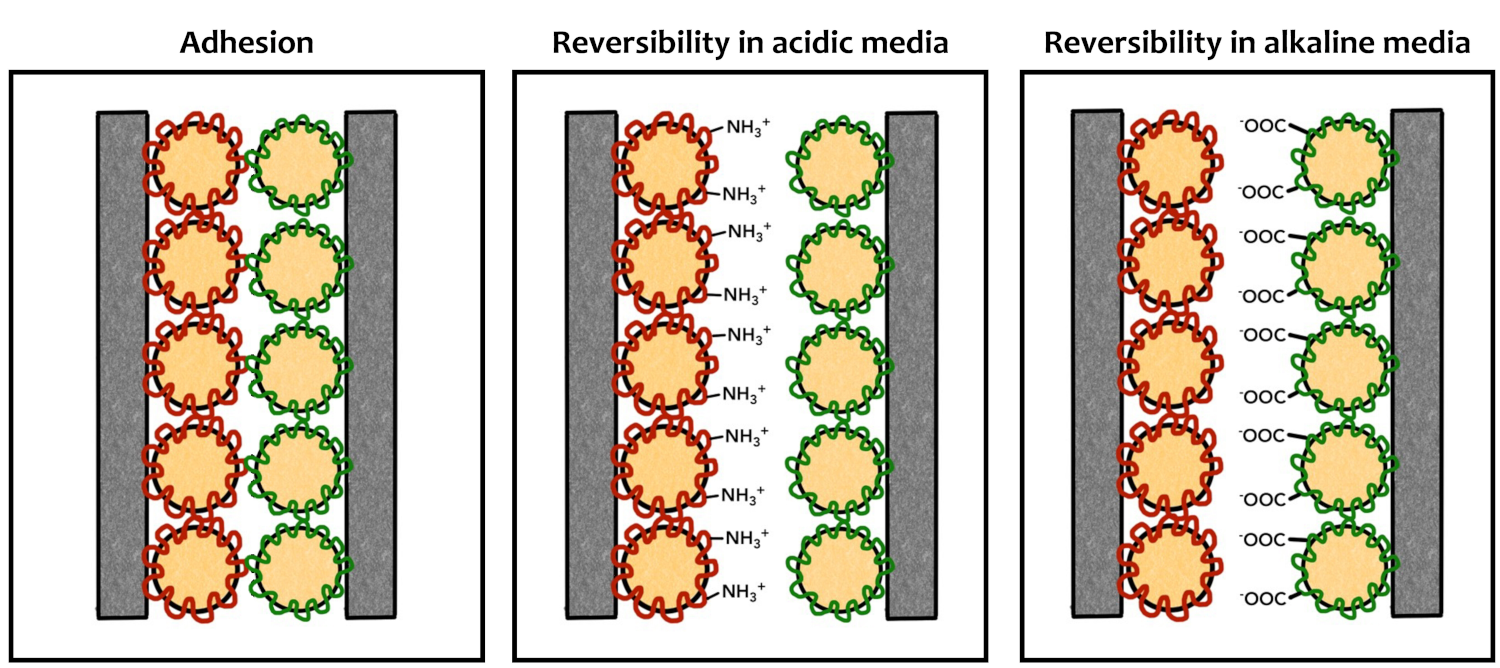
The developed pH-sensitive adhesive system could serve as a recyclable middle ground between structural adhesives with fixed chemical bonds and peel-off adhesive films that bond using physical interactions. The researchers propose integrating a bio-based material from soybean oil into the base polymer: They found that styrene can be substituted by acrylated epoxidized soybean oil (AESO), taking a further step toward environmentally friendly, recyclable bonding systems.
- A reversible water‐based electrostatic adhesive,
Adriana Sierra‐Romero, Katarina Novakovic, Mark Geoghegan,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202310750


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)