Open-shell singlet diradicals are interesting research targets due to their unique electronic, optical, and magnetic properties. Müller’s hydrocarbon, or p,p′-terphenylene-bis-(diphenylmethyl), is an example of a diradicaloid compound with a resonance structure between a closed-shell form and an open-shell diradical form. However, it is highly reactive. More stable analogues could be useful, especially if they have luminescent properties.
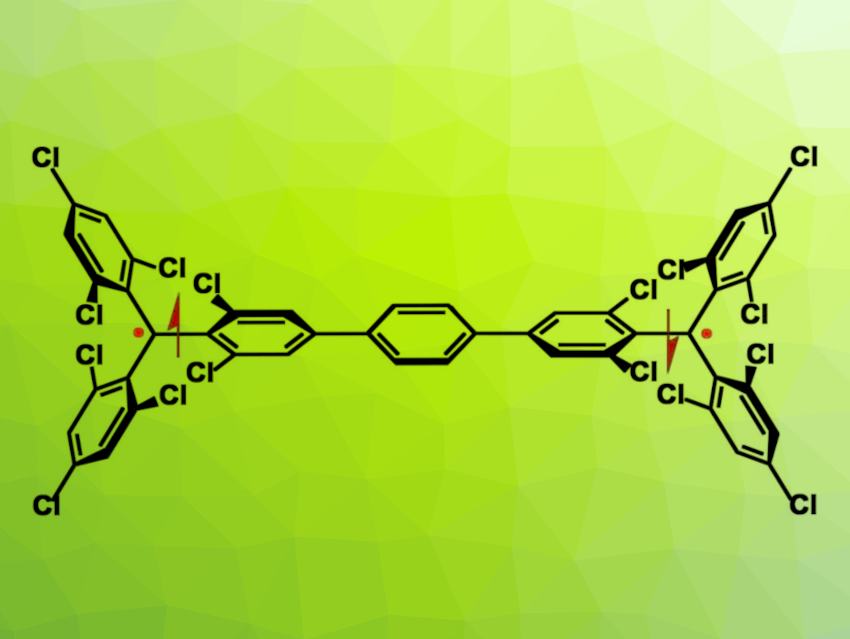
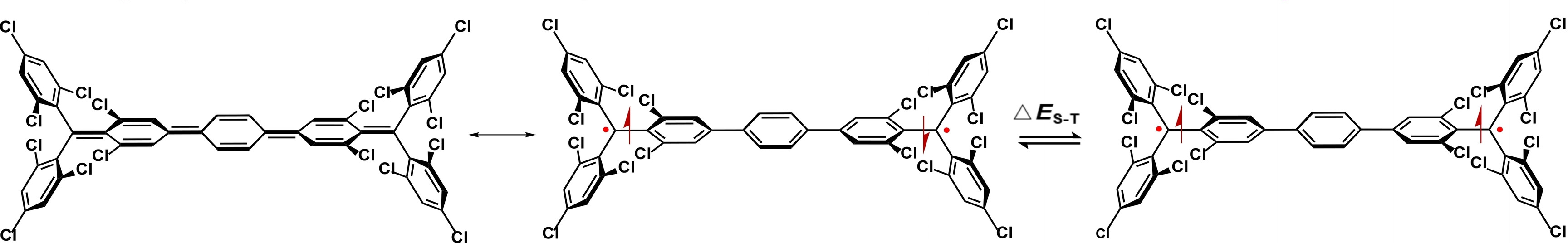
Alim Abdurahman, Jilin University, Changchun, China, Li Shen, Weifang University, China, Qiming Peng, Nanjing Tech University, China, and colleagues have synthesized a stable, luminescent organic diradical based on a Müller’s hydrocarbon structure (named TTM-PhTTM, pictured). The compound has characteristic resonance structures between a closed-shell quinonoid form (pictured below on the left) and an open-shell diradical form. It has an open-shell singlet ground state (pictured below in the center) with a small singlet-triplet energy gap, which enables thermal excitation to a triplet state (pictured below on the right).
TTM-PhTTM was synthesized in three steps from commercially available reagents. First, tris(2,4,6-trichlorophenyl)methane (HTTM) was prepared from 1,3,5-trichlorobenzene and anhydrous chloroform. Two equivalents of HTTM were then reacted with 1,4-benzenediboronic acid bis(pinacol) ester to introduce the bridging phenyl group, followed by oxidation to the desired species. TTM-PhTTM shows high thermal and photostability and has potential for optoelectronic and magnetic applications.
- A Highly Stable Organic Luminescent Diradical,
Alim Abdurahman, Jingmin Wang, Yihan Zhao, Ping Li, Li Shen, Qiming Peng,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202300772