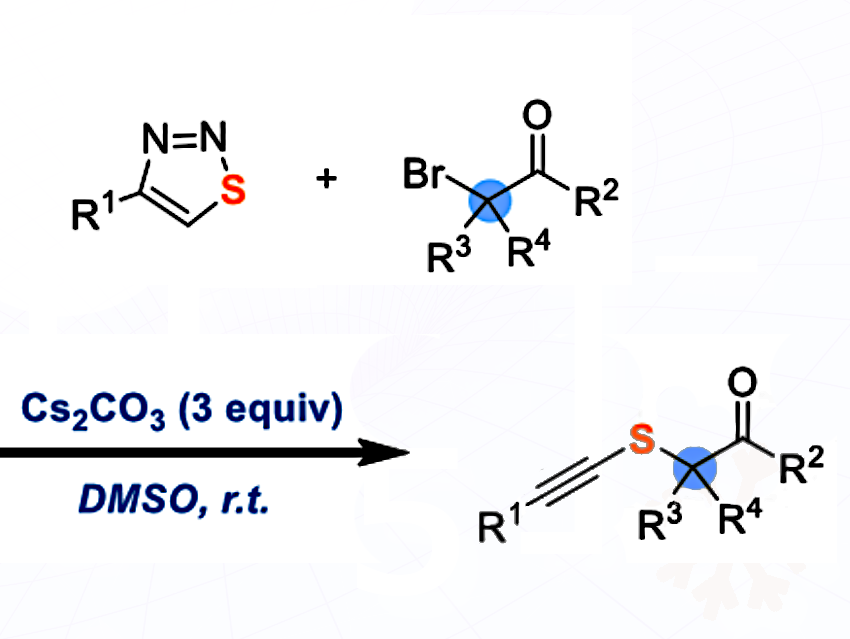
Liangbin Huang, South China University of Technology, Guangzhou, China, and colleagues have developed a transition-metal-free alkynylthiolation of tertiary α-carbonyl bromides with 1,2,3-thiadiazoles to create sulfur-containing tetrasubstituted carbon stereocenters (pictured above). The reaction involves the in-situ generation of alkynylthiolate anions from 1,2,3-thiadiazoles in the presence of a carbonate base, followed by their halogenophilic nucleophilic substitution (SN2X) reactions.
The reaction works under very mild conditions, tolerates many functional groups, and has a broad substrate scope. The reaction runs smoothly on gram scale.
According to the researchers, this provides chemists with a practical, scalable route to complex sulfur compounds, opening doors for new materials and bioactive molecules.
- Transition-Metal-Free Alkynylthiolation of Tertiary α-Carbonyl Bromides
Donghui Xing, Jinxi Huang, Jiaxiang Chen, Liangbin Huang,
Eur. J. Org. Chem. 2025
https://doi.org/10.1002/ejoc.202500618