Hiroshi Shinokubo, Nagoya University, Japan, and his coauthors have discovered a simple reductive dimethylation that transforms Ni(II) 5,15-diazaporphyrins into stable, antiaromatic porphyrinoids with remarkable electron-donating power. The ability of these reduced diazaporphyrins to form charge-transfer complexes highlights a powerful strategy for tuning antiaromatic systems.
What did you do?
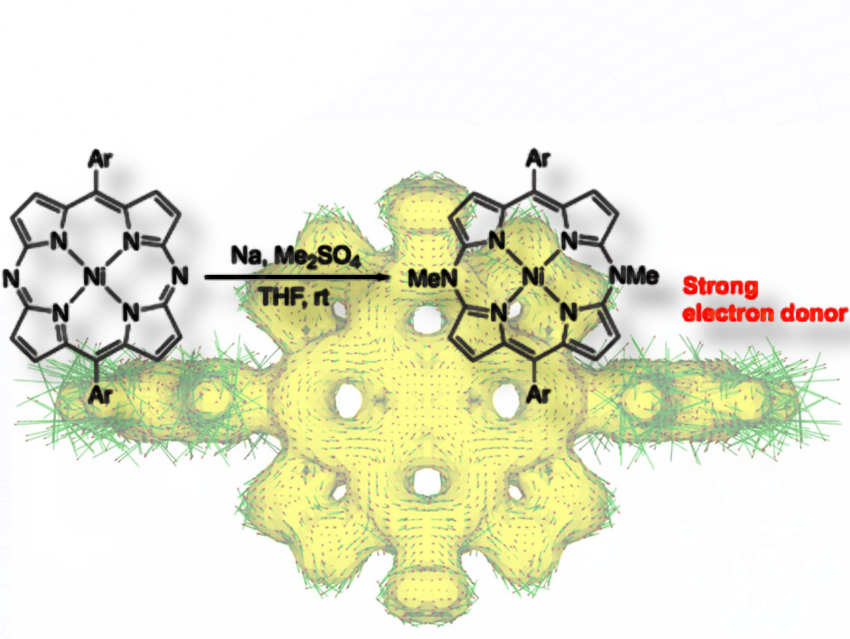
We synthesized stable antiaromatic compounds through a facile two-electron reduction and methylation of aromatic diazaporphyrins, which are porphyrin analogues with two nitrogen atoms substituted at the meso positions.
Why are you interested in this?
Antiaromatic compounds have intriguing properties such as narrow HOMO–LUMO gaps, multi-electron accepting and donating capability, and light absorption in the near IR region. However, they are generally reactive and difficult to isolate.
We need stable antiaromatic compounds to investigate their functions and develop their future applications.
What is new and cool about your work?
To prepare antiaromatic compounds from aromatic compounds, often strong reducing reagents are used, which are generally difficult to handle under ambient conditions. In this work, we employed commercially available sodium dispersion in mineral oil as a user-friendly yet powerful reducing agent to convert aromatic diazaporphyrins into antiaromatic N,N′-dimethyldiazaporphyrins.
What are your key findings?
We demonstrated that reductive dimethylation on the two nitrogen atoms in aromatic diazaporphyrins yields air-stable, antiaromatic N,N′-dimethyldiazaporphyrins in good yields. Electrochemical studies and theoretical calculations revealed that N,N′-dimethyldiazaporphyrins are strongly electron-donating molecules.
What specific applications do you imagine?
Because antiaromatic N,N′-dimethyldiazaporphyrins effectively serve as electron donors, we believe these molecules could be useful for forming charge-transfer (CT) complexes when combined with electron-acceptor molecules. Such CT complexes could be applicable to molecular conductors and magnetic materials in the future.
What part of your work was the most challenging?
Although the present antiaromatic molecules are stable under air, their isolation was initially not so easy because of the oxidation during silica gel column purification. Then, we found that the compounds can be purified by chromatography using a short silica gel column.
Thank you very much for sharing these insights.
The paper they talked about:
- Highly Electron-Donating Antiaromatic N,N′-Dimethyldiazaporphyrins via Reductive Dimethylation,
Kohei Ohtake, Hideaki Takano, Hiroshi Shinokubo,
ChemistryEurope 2025.
https://doi.org/10.1002/ceur.202500109
Hiroshi Shinokubo is a Professor in the Department of Molecular and Macromolecular Chemistry, Graduate School of Engineering, and is affiliated with the Integrated Research Consortium on Chemical Science (IRCCS) and the Research Institute for Quantum and Chemical Innovation, Institutes of Innovation for Future Society, at Nagoya University in Japan.
Also of Interest

Just published articles from ChemistryEurope, the flagship journal of Chemistry Europe