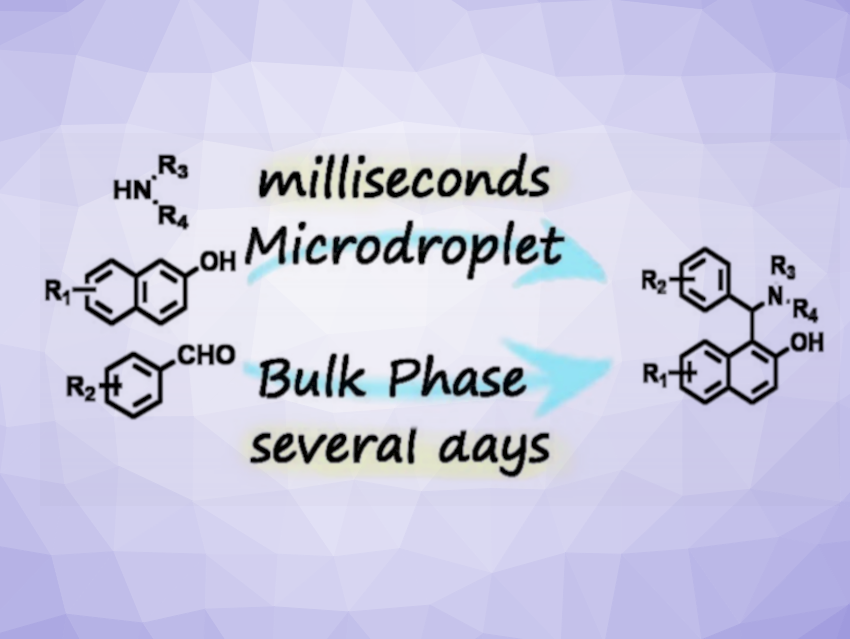
Betti bases (example pictured above on the right) are aminophenolic compounds that have applications, e.g., in pharmaceutical chemistry and catalysis. The tri-component Betti reaction is a classic reaction used to prepare Betti bases from aldehydes, amines, and phenols. These transformations usually require long reaction times under harsh conditions and/or suitable catalysts. New mild, catalyst-free, high-efficiency methods to construct Betti bases would, thus, be useful.
Jinhua Liu, Heyong Cheng, Hangzhou Normal University, China, and colleagues have developed a mild, efficient, and green method to synthesize Betti bases without any catalysts in microdroplets. Microdroplets can accelerate reactions due to their interfacial properties. The reactions were performed in a heated spray chamber, and the microdroplets were created using a nebulizer. The team used a reaction between benzaldehyde, 2-naphthol, and tetrahydropyrrole as a model reaction. They found that optimized yields were achieved using methanol as the solvent, with one equivalent of tetrahydropyrrole, five equivalents of 2-naphthol, and two equivalents of benzaldehyde, as well as a flow rate of 100 μL min−1.
The Betti reaction in microdroplets was accelerated by 6.5⋅103 compared to the bulk reaction. The reaction showed a wide scope for different substituted aldehydes, naphthols, and amines with yields of 68–98 % at a gram-per-hour scale. Overall, the developed approach could be useful for the construction of Betti bases and their derivatives in both laboratory and industrial applications.
- Catalyst‐Free Accelerated Three‐Component Synthesis of Betti Bases in Microdroplets,
Xiaoxiao Jin, Yikang Wu, Chengbiao Dai, Jiannan Sun, Meiying Ye, Jinhua Liu, Heyong Cheng,
ChemPlusChem 2022.
https://doi.org/10.1002/cplu.202200206