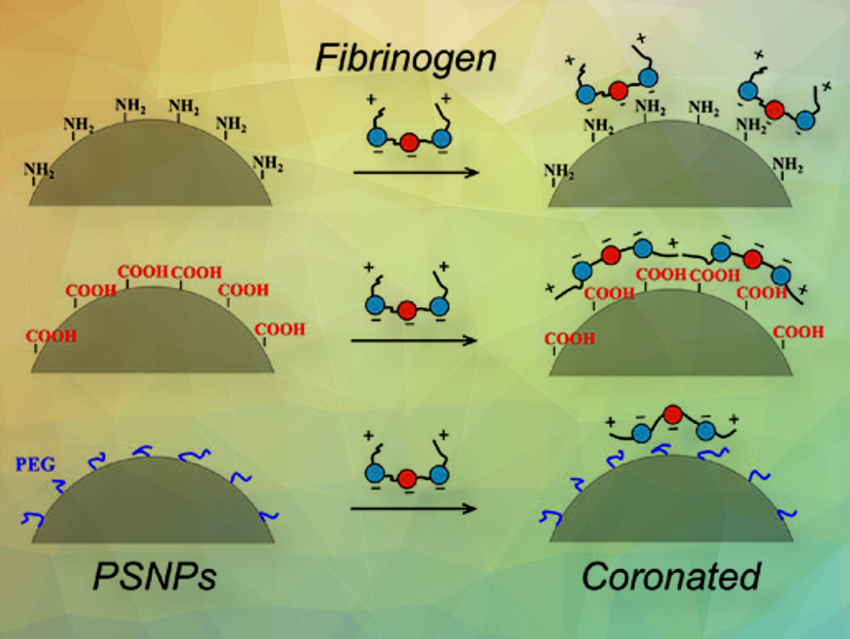
The aggregation of nanoparticles (NPs) induced by plasma proteins is a crucial issue in many biomedical applications. Despite great efforts to study the kinetics of protein adsorption or protein-induced NPs coalescence in bulk solutions, there is limited evidence available for understanding the interfacial circumstances. Changes in diet, disease, medication, or aging can significantly alter the physicochemical properties of the inner lining of blood vessels. In addition, implants such as stents and artificial heart valves have diverse and evolving interfaces. Therefore, it is critical to understand the mechanisms underlying non-specific protein adsorption and NP-protein aggregation in these interfacial scenarios.
Wei Liu, Jiangnan University, China, To Ngai, The Chinese University of Hong Kong, and colleagues have developed a unique surface-sensitive technique called evanescent-light scattering microscopy (ELSM) combined with density-fluctuation theory. Their aim was to study how surface properties influence the structural relaxation, aging process, and dynamic heterogeneity of NP‒protein aggregates at interfaces. The teams findings, based on both time-resolved and spatial-correlated evidence, shows that the aging process of such soft materials is out-of-equilibrium, with dynamics that can show both faster and slower than exponential behavior within a single relaxation process.
Both the protein-to-NP ratio and the surface chemistry of NPs were found to significantly influence the clustering size and morphology of aggregates. Generally, as the protein-to-NP ratio decreased, the degree of aggregation increased, resulting in larger aggregates with fewer proteins. In the case of NP‒fibrinogen (FIB) aggregates formed by polystyrene (PS)-NH2 NPs, a fibril bundle-like structure was observed at low NP concentrations, which became more compact as NP concentration increased. Conversely, for PS-COOH NPs, a porous network composed of interconnected FIB fibers and coronated NP assemblies was observed regardless of the protein-to-NP ratio. Despite PEGylation (covalently attaching polyethylene glycol (PEG)) being commonly believed to repel proteins effectively, the team found that it may not effectively reduce the aggregation degree of polymeric NPs.
This near-surface study provides complementary insights to existing reports on NP‒protein adsorption/aggregation in bulk solutions. These findings could offer valuable guidance for the design of novel antitumor targeted nanomedicines or drug delivery systems for cardiovascular therapies.
- Aggregation and aging of nanoparticle–protein complexes at interfaces studied by evanescent-light scattering microscopy,
Wei Liu, Yuwei Zhu, Hang Jiang, Lidan Zhou, Yinan Li, Jiahao Wu, Jie Han, Cheng Yang, Jianzhong Jiang, To Ngai,
Aggregate 2024.
https://doi.org/10.1002/agt2.538