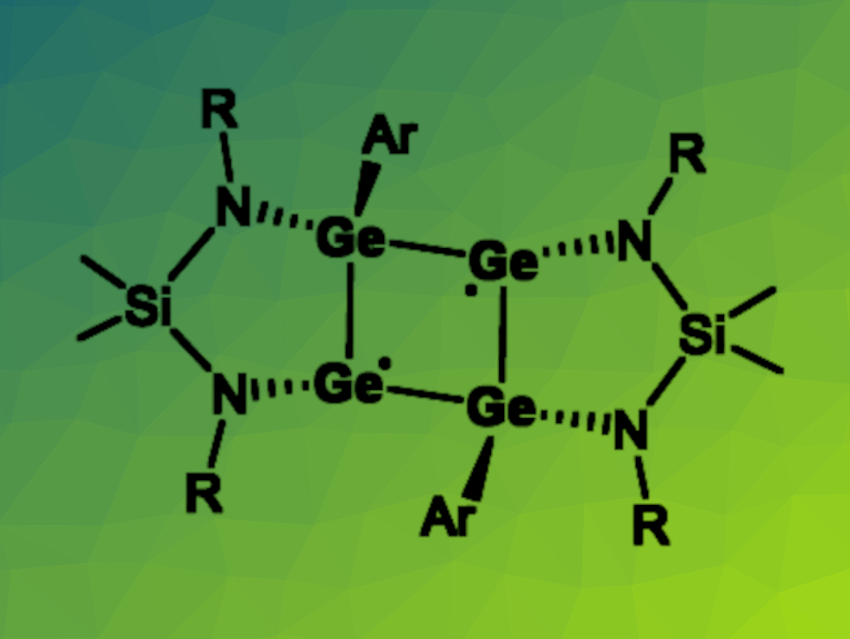
Studying reactive intermediates is an interesting topic in both organic and inorganic chemistry. Cyclobutane-1,3-diyls, for example, are reactive species that could be involved in processes such as the inversion of bicyclo[1.1.0]butanes. Heavier analogues of these four-membered rings with a diradical character can be challenging to synthesize.
Linlin Wu, Central South University, Changsha, China, Hao Wang, Southeast University, Nanjing, China, and Nankai University, Tianjin, China, and colleagues have prepared the first all-germanium analogue of cyclobutane-1,3-diyl (pictured, Ar = 2,6-(2,4,6-Me3-C6H2)2-C6H3, R = 4-Me-C6H). The tetragermacyclobutane-1,3-diyl was prepared by a reduction of a chlorogermylene-coordinated germylgermylene (featuring a four-membered Ge3Cl ring) with potassium graphite in toluene at room temperature.
The desired product was obtained in a yield of 64 % in the form of red crystals. The team characterized the tetragermacyclobutane-1,3-diyl using 1H NMR spectroscopy, which indicated a singlet ground state, UV-Vis spectroscopy, and single-crystal X-ray analysis. They found that the species features a nearly planar Ge4 ring with the two diamidosilane ligands coordinated in a cis-fashion on one side and the two terphenyl groups on the other side. According to the researchers, the isolation of other homonuclear main group analogues of cyclobutane-1,3-diyls is underway.
- Synthesis and Characterization of an All-Germanium Analogue of Cyclobutane-1,3-diyl,
Jiazhong Li, Kai Li, Linlin Wu, Hao Wang,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c13727