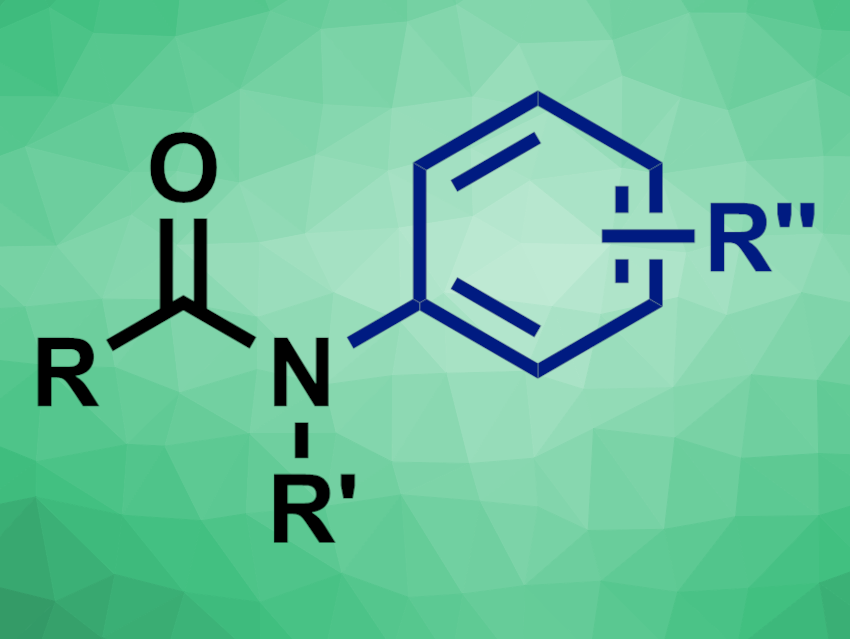
Amide groups are often present, for example, in pharmaceutically active compounds. They can be synthesized, e.g., via coupling reactions of amines and carboxylic acids or rearrangements. Functionalizations of existing primary or secondary amides can be promising alternatives for the preparation of highly functionalized amines. However, amide arylations, for example, generally require organometallic reagents or high temperatures. The comparatively low nucleophilicity of amides makes it challenging to achieve this type of reaction under mild conditions.
Robert D. Bradley and Ana Bahamonde, University of California, Riverside, USA, have found that nickel-photoredox catalysis can be used to promote amide arylations at moderate temperatures and without strong bases (general product structure pictured). The team reacted a broad range of primary or secondary amides with a variety of aryl bromides, using NiCl2·glyme (glyme = dimethoxyethane) as a catalyst together with 4,4′-di-tert-butyl-2,2′-dipyridine (dtbbp) as a ligand, Ir(dtbbpy)(ppy)2PF6 (ppy = (2-pyridinyl)phenyl) as a photocatalyst, K2CO3 as a base, and a mixture of PhCF3 and dimethylformamide (DMF) as the solvent. The reactions were performed at 30 °C under blue LED light.
The desired arylated amines were obtained in moderate to high yields. Other functional groups with similar nucleophilicities, such as phthalimide or sulfonamides, could also be arylated. The mild reaction conditions allow for the use of base-sensitive functional groups. Epimerizable stereocenters are also tolerated without loss of enantiomeric excess. According to the researchers, the strategy could be useful for late-stage functionalizations of amide groups in pharmaceutically active compounds or natural products.
- Mild Amide N-Arylation Enabled by Nickel-Photoredox Catalysis,
Robert D. Bradley, Ana Bahamonde,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02808