The development of new main group species is an interesting research field because such compounds can have interesting bonding situations, properties, and reactivities. Ph3PNNPPh3, for example, can be considered as a donor–acceptor complex of dinitrogen and release N2 upon heating. In low-temperature matrices, species such as FB≡N─N≡BF have been observed. Isolable unsaturated BN compounds could have interesting applications in synthesis.
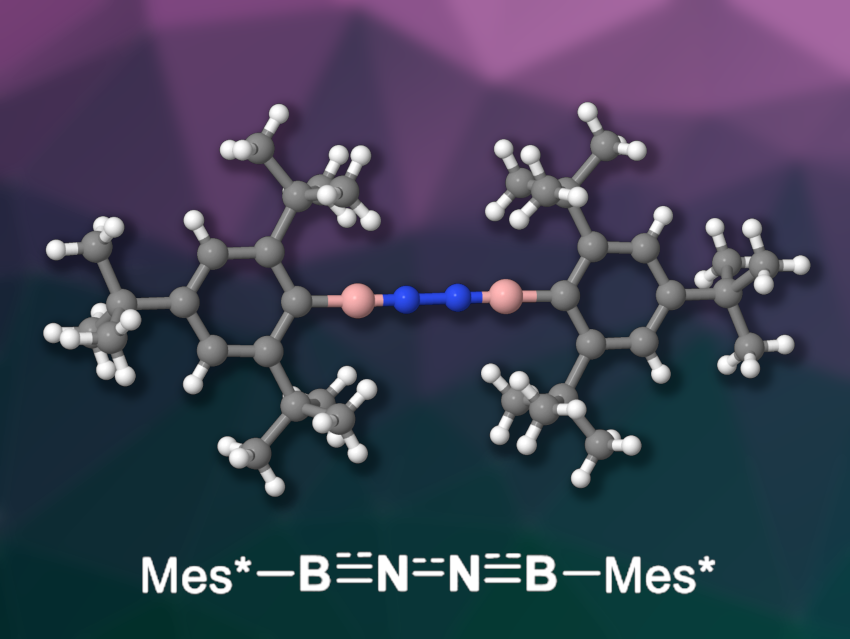
Liu Leo Liu, Southern University of Science and Technology, Shenzhen, China, Lingbing Kong, Shandong University, Jinan, and Nankai University, Tianjin, both China, and colleagues have synthesized and isolated a diiminoborane that is isoelectronic with 1,3-butadiyne (pictured), Mes*B≡N─N≡BMes* (Mes* = 2,4,6-tri-tert-butylphenyl). The team used a dehydrohalogenation reaction: They treated the borylated hydrazine [Mes*B(Br)NH]2 with 1,3-di-tert-butylimidazol-2-ylidene in toluene and obtained the desired diiminoborane in a yield of 40 %.
The product was characterized using NMR, infrared (IR), and UV/Vis spectroscopy, as well as X-ray diffraction. The solid-state structure features an almost linear BNNB unit. Heating the diiminoborane to 50 °C gave an iminoborane with a benzo-fused heterocyclic boryl group. Reacting it with benzonitrile led to a 1,4,2,5-diazadiborinine derivative under loss of N2. The N2 unit can also be reduced to form ammonium ions in a reaction with lithium and aqueous HCl.
- BN Analogue of Butadiyne: A Platform for Dinitrogen Release and Reduction,
Rui Guo, Chaopeng Hu, Qianli Li, Liu Leo Liu, Chen-Ho Tung, Lingbing Kong,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c07469