Rembrandt van Rijn was one of the most important 17th-century Dutch painters. His most famous painting, The Night Watch (1642), hangs in Amsterdam’s Rijksmuseum. Victor Gonzalez, Université Paris-Saclay, Gif-sur-Yvette, France, and Rijksmuseum Conservation & Science, Amsterdam, The Netherlands, and colleagues have identified lead formate—a compound that is very unusual for paintings—in various areas of The Night Watch. The results provide clues about the pictorial practices of Rembrandt and the reactivity of lead driers in the oil matrices of historical paintings.
X-ray Powder Diffraction Mapping
“Operation Night Watch” is a comprehensive research and conservation project in which conservators, art historians, and other scientists across various disciplines are collaborating closely on Rembrandt’s Night Watch. As part of this project, the composition and distribution of materials were examined by macro-X-ray powder diffraction mapping. Synchrotron-based micro-X-ray powder diffraction and infrared microscopy studies were also carried out in parallel on tiny samples. This made it possible for the team to identify and map various lead compounds present in Rembrandt’s paint layers.
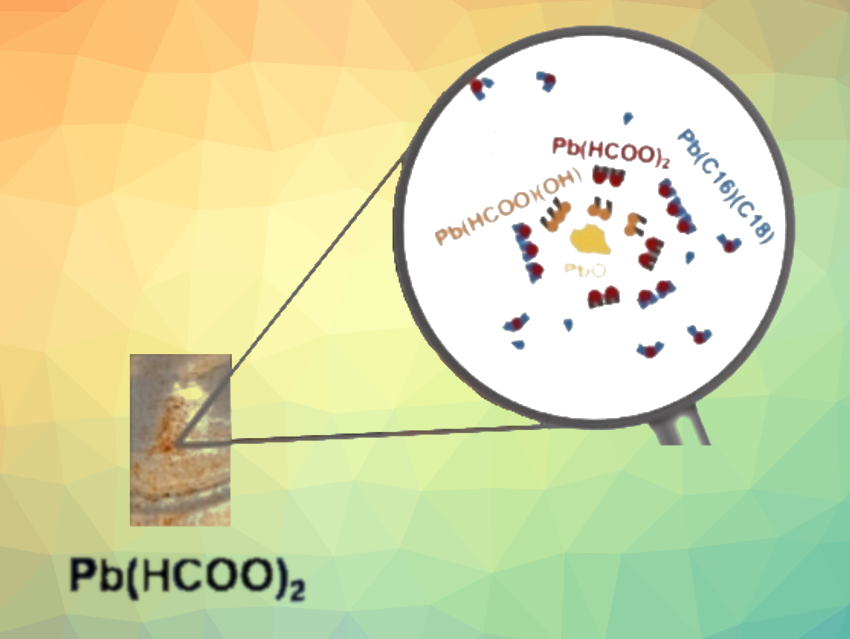
Lead pigments were widely used by Rembrandt. The most common was white lead, a mixture of lead carbonates hydrocerussite (Pb3(CO3)2(OH)2) and cerussite (PbCO3). Lead is also present in other pigments and their associated alteration products. However, one discovery made by the team was more unusual: lead(II) formate (Pb(HCOO)2)—a compound that has never before been found in historical oil paintings. Lead formate, the lead salt of formic acid, was found in several areas of The Night Watch—sometimes together with plumbonacrite (Pb5(CO3)3O(OH)2), another rare lead compound.
Clues on the Reactivity of Metallic Driers
To investigate the chemical origin of lead formate, the team produced model paint layers according to old recipes. For example, siccative oil was prepared by heating linseed oil, the most common binding agent for paints at the time, with lead oxide (PbO). Lead oxide is a metallic dryer, causing paints to harden more quickly.
The study showed that PbO in oil paint can react to form lead formate. Even though no crystalline PbO was detected in The Night Watch, the results support the hypothesis that an oil containing such a lead dryer was used. However, other hypotheses also have to be considered. Past conservation works on The Night Watch, notably the possible addition of an oil-based varnish in the 18th century, could have favored the formation of lead formate on the painting. The team is now investigating the kinetics of the formation of lead formate and associated compounds, as well as their stability in oil paint.
- Lead(II) Formate in Rembrandt’s Night Watch: Detection and Distribution from the Macro‐ to the Micro‐scale,
Victor Gonzalez, Ida Fazlic, Marine Cotte, Frederik Vanmeert, Arthur Gestels, Steven De Meyer, Fréderique Broers, Joen Hermans, Annelies van Loon, Koen Janssens, Petria Noble, Katrien Keune,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202216478