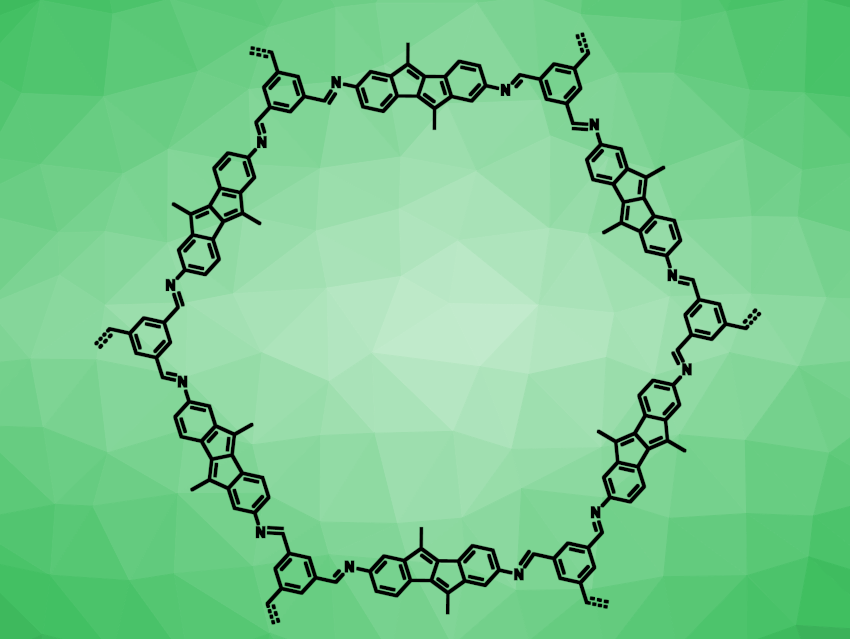
Polycyclic aromatic molecules are useful, e.g., in organic materials. In contrast to aromatic compounds with 4n+2 conjugated π-electrons, antiaromatic compounds with 4n conjugated π-electrons are destabilized, which hampers their application. Nevertheless, antiaromatic systems can have useful optical and electronic properties. The annellation of aromatic rings to antiaromatic compounds can be used to stabilize them. For example, the antiaromatic pentalene can be stabilized by benzannellation, giving dibenzo[a,e]pentalene (DBP).
Birgit Esser, Ulm University, Germany, Oliver Dumele, Humboldt University of Berlin, Germany, and colleagues have prepared a series of imine-linked two-dimensional covalent organic frameworks (COFs) which contain DBP subunits (example pictured). The team prepared three different DBP-based building blocks, with either 2,7-substitution (MeDBP and PhDBP) or 5,10-substitution (DBP-Ph) for the linking sites. These building blocks were linked using triformylbenzene (TFB) or triformylphloroglucinol (TFP) in solvothermal syntheses. The team obtained three crystalline porous COFs (MeDBP-TFB COF, PhDBP-TFP COF, and DBP-Ph-TFB COF) and two amorphous porous polymers.
According to the researchers, the products are the first framework materials prepared from antiaromatic building blocks. They show interesting optical and electronic properties, for example, broad optical absorption, photoconductivity upon irradiation with visible light, and useful redox properties for battery applications.
- Antiaromatic Covalent Organic Frameworks Based on Dibenzopentalenes,
Josefine Sprachmann, Tommy Wachsmuth, Manik Bhosale, David Burmeister, Glen J. Smales, Maximilian Schmidt, Zdravko Kochovski, Niklas Grabicki, Robin Wessling, Emil J. W. List-Kratochvil, Birgit Esser, Oliver Dumele,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.2c10501