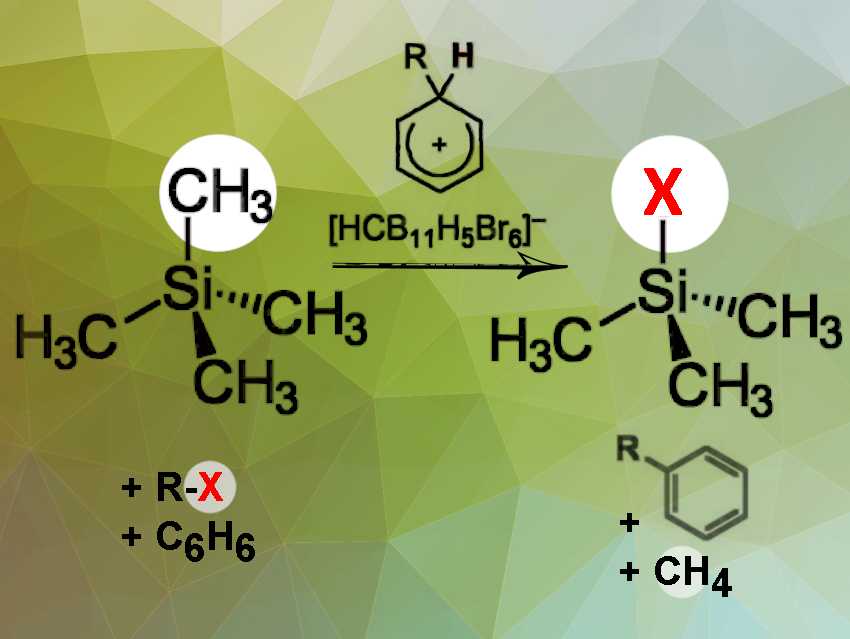
The preparation of silanes and their targeted further processing is not trivial. A typical starting point is chlorosilanes such as Me4-nSiCln (n = 1–3) and SiCl4 (n = 4). Unfortunately, it is often difficult to separate the components. On the other hand, the preparation of silanes with four alkyl groups is generally considered a synthetic dead end.
Martin Oestreich, Hendrik F. T. Klare, and Tao He, Technische Universität Berlin, Germany, have developed a method using arenium-ion-catalyzed halodealkylation to effectively transform Me4Si and related quaternary silanes into a wide variety of functionalized derivatives. This is done in one pot in two steps, protodealkylation and then halogenation. The team uses an alkyl halide and an arene (co)solvent in this process (pictured above). The alkyl halide serves as the halide source, which eventually participates in a Friedel–Crafts alkylation with the arene to regenerate the catalyst. The arenium ion acts as a strong Brønsted acid during the protodealkylation step.
The team demonstrated the advantages of their top-down halodealkylation approach (starting from fully alkylated silanes) over previously reported bottom-up procedures (starting from functionalized silanes) in practical applications by exemplarily synthesizing a silicon drug precursor. Furthermore, they showed that this selective chlorodemethylation of the relatively unreactive Me3Si group attached to an alkyl chain, followed by oxidative degradation, provides a pathway for Tamao–Fleming-type alcohol formation.
- Arenium-ion-catalysed halodealkylation of fully alkylated silanes,
Tao He, Hendrik F. T. Klare, Martin Oestreich,
Nature 2023.
https://doi.org/10.1038/s41586-023-06646-9