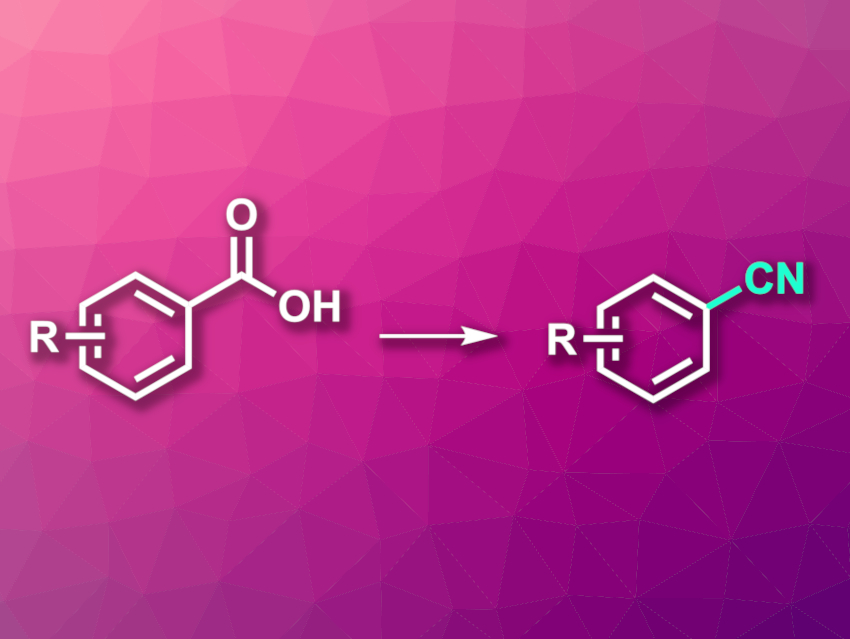
Aromatic nitriles are useful intermediates in organic synthesis and are also often found in, e.g., bioactive molecules or agrochemicals. Traditional approaches to the synthesis of aromatic nitriles are often hampered by a need for toxic reagents or narrow substrate scopes. Carboxylic acids are widely available and can be useful substrates for the formation of new C–C bonds via reactions involving decarboxylations or decarbonylations.
Chengrong Ding, Zhejiang University of Technology, Huzhou, China, and colleagues have developed a method for the preparation of aryl nitriles via a palladium-catalyzed decarbonylative cyanation of aryl carboxylic acids (overall reaction pictured). The team reacted a wide range of aryl carboxylic acids with Zn(CN)2 as a nitrile source, using trimethylacetic anhydride (pivalic anhydride, Piv2O) as an activator and Pd(OAc)2 as the catalyst together with 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (Xantphos) as a ligand. The reactions were performed in dioxane at 160 °C.
The desired aryl nitriles were obtained in moderate to excellent yields. The researchers demonstrated the scalability of the reaction by a synthesis of 2-naphthonitrile on a gram scale with a yield of 92 %. According to the team, the protocol does not require the addition of a base, shows good functional group tolerance, and can also be used to modify bioactive molecules.
- Palladium-Catalyzed Direct Decarbonylative Cyanation of Aryl Carboxylic Acids,
Guofu Zhang, Huihui Miao, Chenfei Guan, Chengrong Ding,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.2c01401