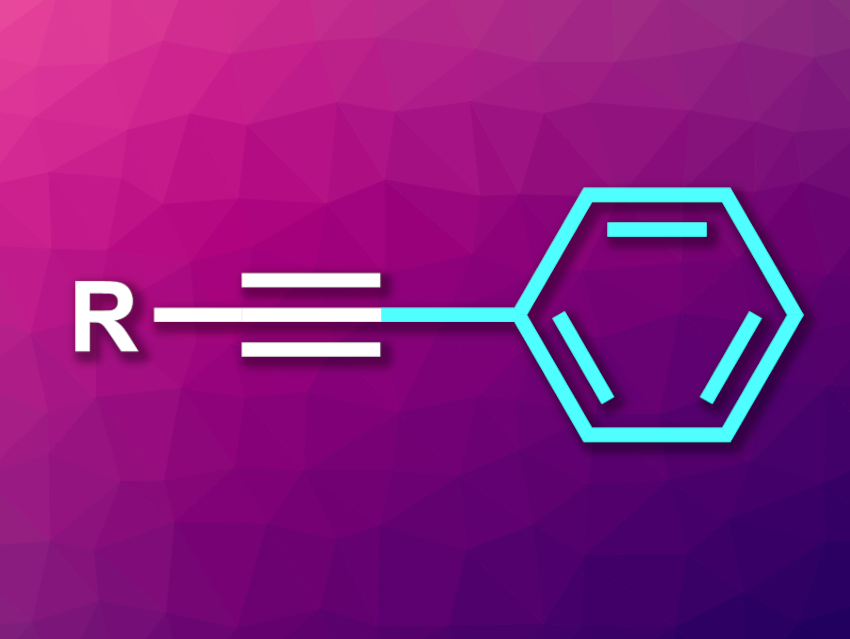
Alkynes are useful building blocks in organic synthesis and also have uses, e.g., in materials science. Aryl alkynes, for example, can also occur in natural products and bioactive compounds. There are transition-metal-catalyzed methods for the construction of C(sp)–C(sp2) bonds to make aryl alkynes, as well as some transition-metal-free alternatives. Nevertheless, the development of additional convenient and efficient transition-metal-free methods for the synthesis of aryl alkynes would be useful.
Qiuling Song, Fujian Province University, Fuzhou, China, and Henan Normal University, Xinxiang, China, and colleagues have developed a transition-metal-free Sonogashira-type coupling for the one-pot arylation of terminal alkynes (general product structure pictured). The approach is based on the removal of the acetylenyl hydrogen using n-BuLi, followed by the addition of pyridine triphenylborate to give an alkynyl triphenylborate intermediate and an N-iodosuccinimide (NIS)-mediated transformation to give the desired aryl alkyne.
Using this three-step, one-pot approach, the team arylated a variety of terminal alkynes, both aromatic and aliphatic. The method showed good functional group compatibility, and the desired products were obtained in moderate to excellent yields. The team also successfully used other boranes, including a trialkylborane and triarylboranes with electron-withdrawing or electron-donating groups. The researchers propose that the reaction mechanism involves an electrophilic addition of I+ to the tetracoordinate boron intermediate, followed by a migration and a syn-elimination to re-form the triple bond.
- Arylation of Terminal Alkynes: Transition-Metal-Free Sonogashira-Type Coupling for the Construction of C(sp)–C(sp2) Bonds,
Mingxing Ye, Mengyuan Hou, Yahao Wang, Xingxing Ma, Kai Yang, Qiuling Song,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c00586