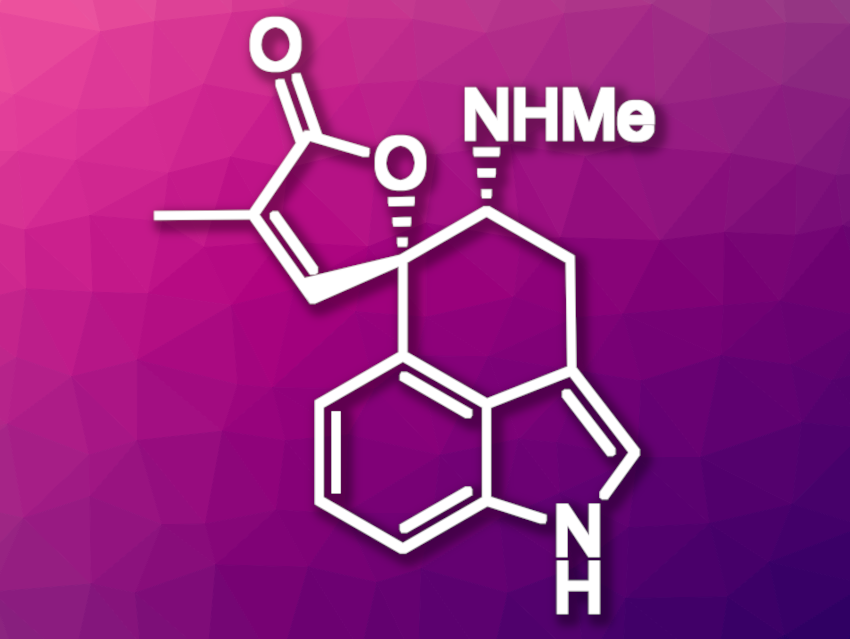
Clavine alkaloids are indole derivatives produced by several families of fungi. There is only a limited number of detailed studies concerning the biological and toxicological properties of naturally occurring clavine alkaloids, likely due to insufficient amounts of material. An efficient chemical synthesis of natural clavines would be useful in this context. Rugulovasine A (pictured above) and B are examples of tetracyclic clavine alkaloids.
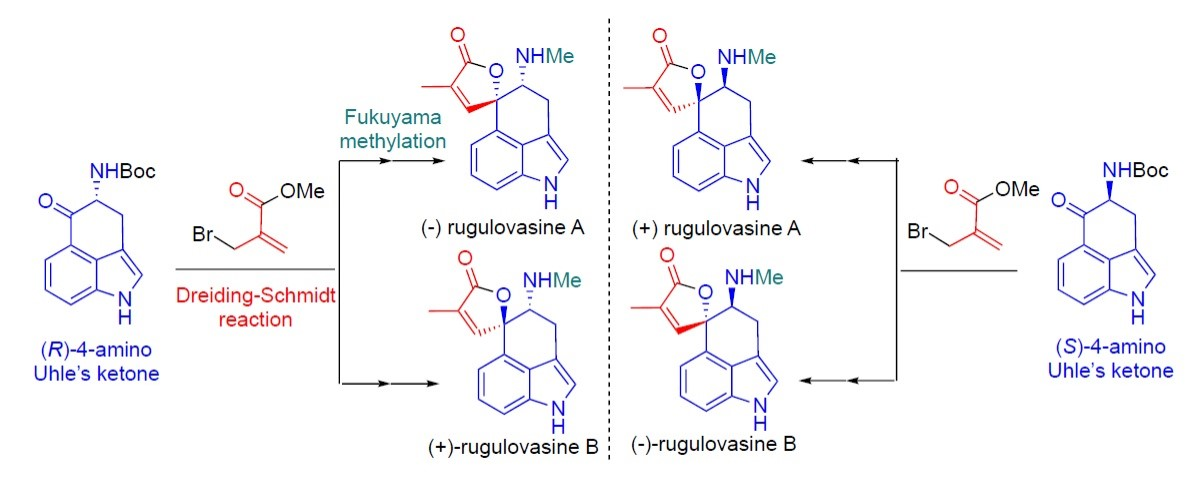
Giovanni Piersanti, University of Urbino, Italy, and colleagues have developed a unified enantioselective synthesis of all four rugulovasine stereoisomers (pictured below) as single enantiomers, including the first total syntheses of (−)- and (+)-rugulovasine B. In all four syntheses, the team used a 4-amino Uhle’s ketone and a methacrylate derivative to create the unsaturated oxaspirolactone unit using a Dreiding–Schmidt reaction. This was followed by a Fukuyama methylation to give the desired N-methyl secondary amines.
The researchers then tested all enantiopure stereoisomers of rugulovasine for their binding affinities to dopamine, serotonin, and adrenergic neuroreceptors. They found selectivity for serotonin receptors over dopamine and adrenergic receptors and excellent affinity for the 5-HT1A receptor (a receptor that binds serotonin). (+)-Rugulovasine B was the most interesting isomer in terms of its effective concentration and serotonin receptor selectivity.
Based on this work, all four enantiopure rugulovasine stereoisomers are synthetically available. This could allow further evaluation of this clavine natural product.
- Asymmetric Total Synthesis of All Rugulovasine Stereoisomers and Preliminary Evaluation of their Biological Properties,
Francesca Bartoccini, Alessio Regni, Michele Retini, Giovanni Piersanti,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200315