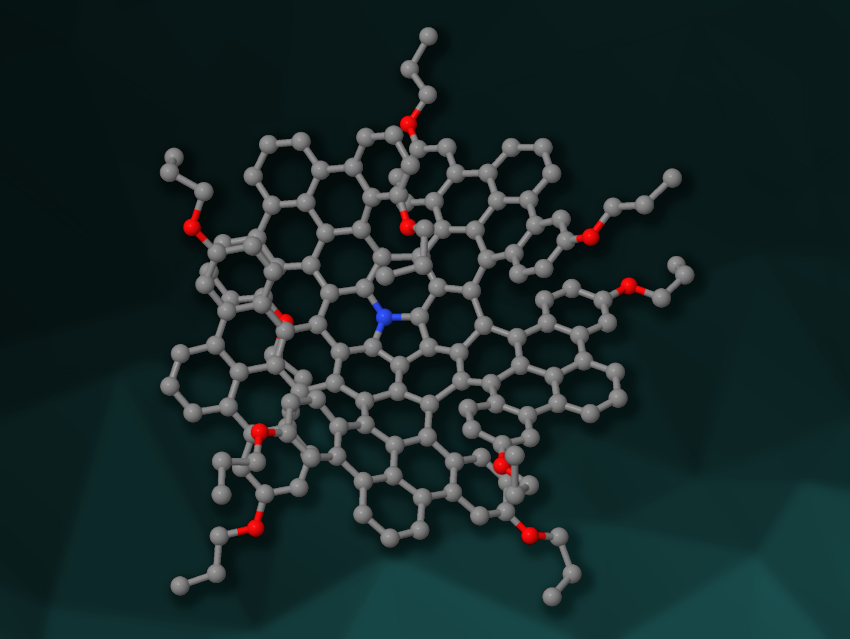
Helicenes are chiral, non-planar polycyclic aromatic hydrocarbons. They have potential applications, e.g., in organic electronics or molecular machines. Combining multiple helicene units in one molecule can lead to interesting and useful new properties. The helicene units can be connected, for example, using planar hexabenzocoronene or non-planar corannulene cores. Replacing these structures with other cores could allow researchers to tune the compounds’ properties.
Qianyan Zhang, Xiamen University, China, and colleagues have designed and synthesized a new azabuckybowl-helicene hybrid π-system (simplified structure pictured), called N-Q7H. This compound contains five [7]helicene subunits: two aza[7]helicenes and three [7]carbohelicenes. These helicenes are created from an azapentabenzocorannulene core and five dibenzopyrene “propeller blades”.
N-Q7H was synthesized in three steps from a known azapentabenzocorannulene, which was borylated with bis(pinacolato)diboron (B2(pin)2), followed by a five-fold Suzuki–Miyaura coupling using 2′-iodo-3,”-dipropoxy-1,1′:3′,1”-terphenyl. An intramolecular cyclodehydrogenation then gave the desired product, which is soluble in common organic solvents.
Single crystal X-ray analysis of N-Q7H showed its propeller-like shape with five “blades” and a central azacorannulene bowl with a depth of 0.76 Å. According to the researchers, N-Q7H is the first example of a multiple helicene with an azabuckybowl core. It showed broad NIR absorption and an NIR fluorescence quantum efficiency of 28 %.
- Nitrogen-Embedded Quintuple [7]Helicene: A Helicene–Azacorannulene Hybrid with Strong Near-Infrared Fluorescence,
Yin-Fu Wu, Si-Wei Ying, Li-Yun Su, Jun-Jie Du, Ling Zhang, Bin-Wen Chen, Han-Rui Tian, Han Xu, Mei-Lin Zhang, Xiaomei Yan, Qianyan Zhang, Su-Yuan Xie, Lan-Sun Zheng,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c00794