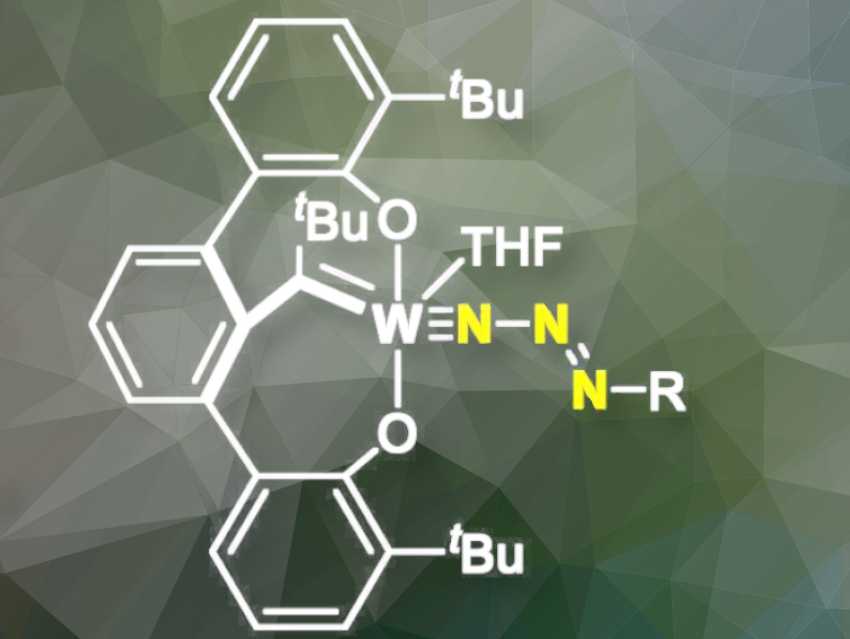
Metal complexes bearing an azoimido (M-N3R) ligand are thermodynamically unstable and easily convert into the corresponding imido (M-NR) complexes, releasing dinitrogen in the process. In catalysis, nitrene transfer is the most common use of these fleeting azoimido intermediates. However, catalysts containing an azoimido that does not decompose during catalysis are unknown, until now.
Adam S. Veige, University of Florida, Gainesville, FL, USA, and colleagues have synthesized tethered tungsten alkylidene complexes bearing stable spectator azoimido ligands (M≡Nγ-Nβ=NαR) in high yields.
The team tested them as initiators for ring expansion metathesis polymerization (REMP). Typically these ligands are unstable and prone to dinitrogen loss, but this work demonstrates that tethered alkylidene complexes bearing azoimido ligands are stable enough to be REMP initiators. These W-azoimido complexes are kinetically stable, converting to the corresponding imido complexes with a half-life of >400 minutes, even at 90 °C. Kinetic experiments and Density Functional Theory (DFT) computational studies suggest that both steric and electronic factors play crucial roles in the stabilization of the azoimido complexes.
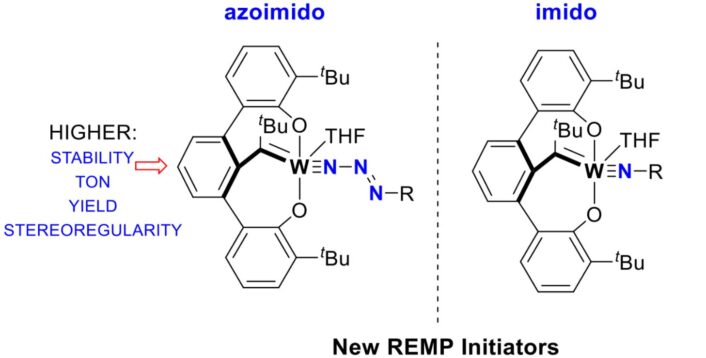
Exploiting the high stability, the researchers demonstrate that the azoimidos initiate ring expansion metathesis polymerization (REMP) to synthesize cyclic polymers. They found that the azoimido lignds are more efficient, long-lived, and stereoselective than their corresponding imido derivatives (M≡NR). The team demonstrates that the azoimido catalysts are three orders of magnitude more active than catalysts with ubiquitous imido ligands.
- REMP Initiators with an Unusual Ancillary Ligand,
Rinku Yadav, Alec Esper, Ion Ghiviriga, Khalil Abboud, Daniel Lester, Christian Ehm, Adam S. Veige,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202301678