Bacteria that feed on methanol can grow on certain rare earth elements as well as their radioactive relatives. These findings suggest a possible role for such bacteria in the decontamination of areas where actinides are spilled, or in the separation of lanthanides and actinides for analytical or preparative purposes, according to work by Lena J. Daumann, Ludwig-Maximilians-University Munich, Germany, and colleagues.
Actinides Replacing Lanthanides in the Metabolism of Bacteria
Lanthanides belong to the rare earth elements that are widely used in electronics and energy technologies. With one exception, they are not radioactive, they are abundant in the Earth’s crust, and some lanthanides such as lanthanum and neodymium even play a crucial role in bacterial metabolism. Actinides, which include uranium and plutonium, are their heavy, radioactive counterparts. The team discovered that some actinides can replace essential lanthanides in the metabolism of methylotrophic bacteria.
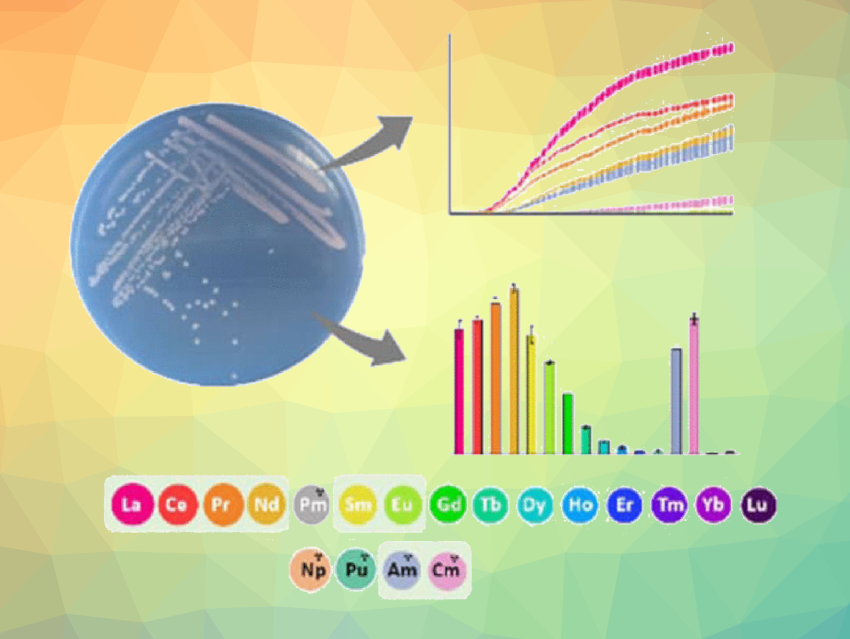
Methylotrophic bacteria, so-called because they use methanol as their energy source, contain lanthanides in their methanol-oxidizing enzyme. The researchers performed growth studies in the presence of actinides using two strains of bacteria: the volcanic methanotroph Methylacidiphilum fumariolicum SolV and the Methylobacterium extorquens AM1 ΔmxaF mutant. They ruled out lanthanide impurities that could have otherwise stimulated growth and used liquid scintillation counting and inductively coupled plasma mass spectrometry (ICP-MS) to monitor metal concentrations in the bacteria’s growth medium.
The researchers also measured the enzymatic activity of the lanthanide-dependent enzyme in the presence of various metal ions in vitro. The enzyme oxidizes methanol to formaldehyde, which was observed using a dye-coupled assay.
The team chose three actinides as potential lanthanide replacements because they display similar properties to the light lanthanides: Americium and curium, which are stable in the +III oxidation state and have a matching size to Nd3+, and plutonium, which is similar to Pr3+ in the +III oxidation state, but has a richer redox chemistry.
Sizes and Oxidation States Are Important
The team observed that the bacteria could take up actinide ions into their methanol-oxidizing enzyme, using them for methanol metabolism in the same way as they would use lanthanides. This observation held particularly well when the actinide ions were the same size and had the same stable +III oxidation state as the corresponding lanthanides. The bacteria even preferred taking up the actinides americium and curium over the later lanthanides when presented with a mixture of lanthanides and actinides, which is due to the sizes of the respective ions.
A stable +III oxidation state appeared to be particularly important. A lack of enzyme activity and bacterial growth with plutonium under the (aerobic) experimental conditions hints towards the importance of the oxidation state for the enzymes and strains tested in this work. “When we used plutonium, which is known to have higher oxidation states, our bacteria did not grow, nor did the isolated enzymes work with it either,” Daumann says.
Possible Applications
Actinides such as plutonium, americium, and curium can be generated in nuclear reactors and are used in research, the nuclear industry, and many other technologies. Americium is even a source of ionizing radiation in commercial smoke detectors. However, actinides are highly radioactive and hazardous elements, which have to be handled with great care at special facilities. Spillages of radioactive substances are always of great concern.
Dauman proposes “putting these bacteria to use to help clean up radioactive spills” as a possible future application. The team also plans to explore the ability of the bacteria to extract actinides from radioactive waste or to separate out specific elements from mixtures. Bacteria that thrive at low pH values could, for example, be useful in the recycling of lanthanides and actinides from end-of-lifetime products, industrial feedstocks, or metal leachates.
- Minor Actinides Can Replace Essential Lanthanides in Bacterial Life,
Helena Singer, Robin Steudtner, Andreas S. Klein, Carolin Rulofs, Cathleen Zeymer, Björn Drobot, Arjan Pol, N. Cecilia Martinez‐Gomez, Huub J. M. Op den Camp, Lena J. Daumann,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202303669