Mahdi Alizadeh and colleagues from Aalto University School of Science and Technology in Espoo, Finland, have modeled and designed an “electrolyte-free fuel cell” using a GaAs diode. The cell can facilitate chemical reactions involving the oxidation of fuel and the reduction of an oxidizing agent directly on the bands of the semiconductor material it contains, specifically both its conduction band (where electrons are free to move and conduct electricity) and its valence bands (where electrons are normally bound to atoms). This is how it converts renewable chemical energy and light into electricity.
Dr. Alizadeh, what did you do?
We fabricated a GaAs pn-junction similar to those used in GaAs solar cells and exposed an active surface of the device to an organic fuel or fuel vapors, in this case methanol. It turned out that the semiconductor mediated the oxidation of the fuel and became electrically excited in the process. This excitation was then observed as an external current and voltage generated by the diode, allowing direct conversion of the chemical energy of the fuel into electricity.
To provide a reasonable explanation for the observations, we also developed a theoretical model describing the excitation, based on models previously developed for semiconductor electrochemistry.
Why are you doing this?
First, we wanted to know if it was possible to directly convert chemical energy from renewable fuels into electricity using semiconductor materials and widely available solutions from semiconductor technologies.
Second, we wanted to understand what mechanisms might make this possible and what currently limits the efficiency and power of the process. In the longer term, we hope that different catalysts and surface passivation methods could be used to explore a new avenue for sustainable energy conversion using hybrid solar cell devices.
What is new and cool about it?
Our research demonstrates the “chemovoltaic effect”, which is the chemical equivalent of the photovoltaic effect. In the chemical effect, exergonic redox reactions on semiconductor surfaces generate electronic excitation, allowing solar cells to be used as hybrid devices that can also feed on chemical energy. This process enables the direct conversion of chemical energy from methanol (in both liquid and vapor phases) into electricity without the need for additional electrolytes.
The approach has revolutionary potential to overcome the limitations of conventional fuel cells, which require two electrodes, ionic conduction, and semi-permeable membranes, while our approach would require none of these and allow energy conversion also through the gas phase.
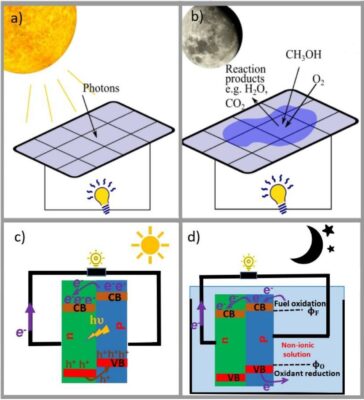
Figure 1. a) and c) show photovoltaic cells that produce electron-hole pairs and electrical energy from light. b) and d) illustrate chemovoltaic cells, which uses carriers injected from chemical reactions of a redox couple, rather than relying on light to function.
What is the main significance of your results?
Our results demonstrate the feasibility of using semiconductor-based chemovoltaic cells to directly convert chemical energy from renewable fuels into electricity. This may open up a new research topic and opportunities for the development of sustainable energy conversion devices or chemical detectors.
What specific applications do you envision?
In the best case, we envision practical applications such as hybrid chemo-/photovoltaic cells capable of providing all-day power from renewable chemical energy sources in the absence of light. Achieving this longer-term vision would require optimizing cell designs, exploring new materials, identifying suitable catalysts and fuels, and potentially integrating these cells into various energy systems to improve sustainability.
What was the most challenging part of your work?
The most challenging aspects of the work so far have been finding the device structures that behave reliably enough to consistently observe the relatively small currents and voltages, and convincing various reviewers that the work makes sense and that our hypothesis about the effects is feasible. In the next steps, the challenges will likely shift to increasing the effectiveness of the approach by developing suitable catalysts and surface treatments for the cells.
Thank you very much. We wish you all the best.
The paper they talked about:
- Chemovoltaic effect for renewable liquid and vapor fuels on semiconductor surfaces,
Mahdi Alizadeh, Ivan Radevici, Shengyang Li, Jani Oksanen,
ChemSusChem 2024.
https://doi.org/10.1002/cssc.202301522

Dr. Mahdi Alizadeh is a Postdoctoral Research Fellow at Engineered Nanosystems Group, School of Science, Aalto University, Espoo, Finland.