Mn(III) species are useful in different reactions and important in biological systems. However, there are only a few Mn(III) precursors that are convenient for use in the laboratory. MnCl3, for example, is thermally unstable and needs to be freshly prepared, often using impractical reactions.
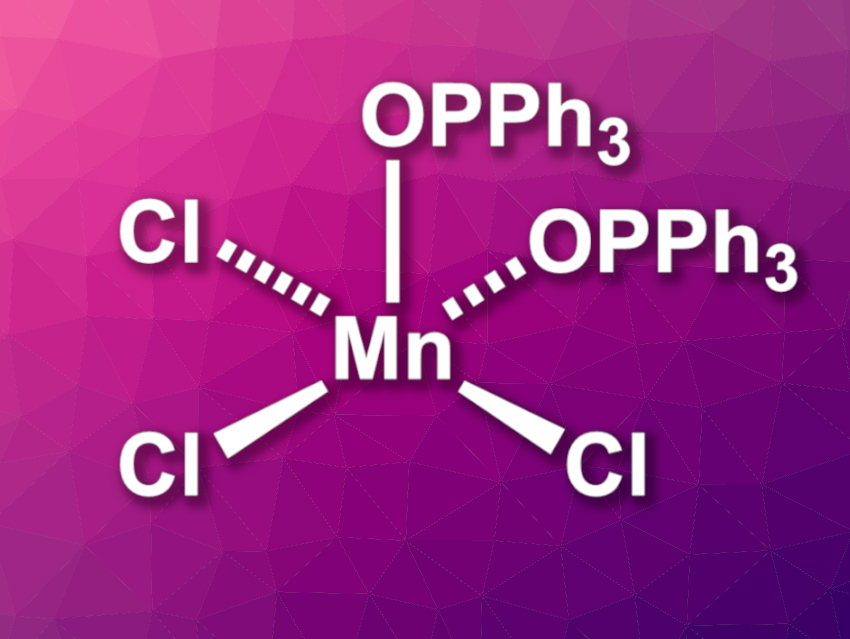
David C. Lacy, University at Buffalo, State University of New York, USA, and colleagues have investigated the complex [MnCl3(OPPh3)2] (pictured), which is easy to prepare and bench-stable. This complex can serve as a convenient source of MnCl3. The team prepared the complex by treating [Mn12O12(OAc)16(H2O)4]·2HOAc·4H2O with Me3SiCl in MeCN to give solvated MnCl3, which was then reacted with Ph3PO to obtain the desired complex as a precipitate. The same product can also be obtained directly from Mn(OAc)2, KMnO4, and Me3SiCl in a one pot process by treating a mixture of these substrates in MeCN with Ph3PO, albeit in slightly lower yields.
The prepared complex is stable under ambient conditions in solid form. It was characterized using X-ray diffraction, and the team found that [MnCl3(OPPh3)2] has a square-pyramidal structure. The Ph3PO units can be substituted by other ligands, which is useful in coordination chemistry. As an example, the researchers prepared the complex {Tpm*}MnCl3 (Tpm* = tris(3,5-dimethyl-1-pyrazolyl)methane) via ligand substitution. [MnCl3(OPPh3)2] also has applications in organic synthesis. It can be used as a chlorine atom transfer agent for alkene dichlorination reactions with a broad substrate scope.
- Synthesis of a Bench-Stable Manganese(III) Chloride Compound: Coordination Chemistry and Alkene Dichlorination,
Ananya Saju, Justin R. Griffiths, Samantha N. MacMillan, David C. Lacy,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08509