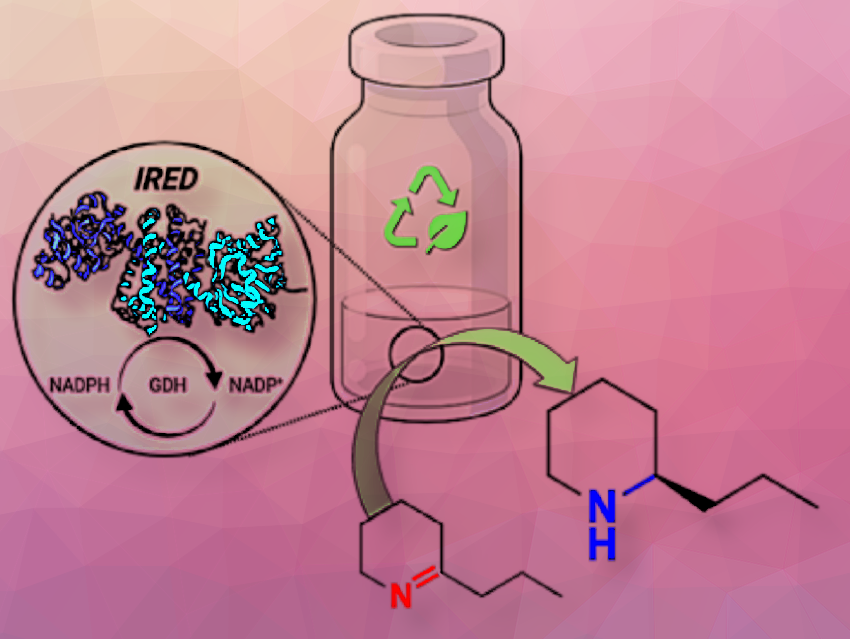
Biocatalysis is a sustainable and green approach to chemical transformation. However, challenges related to the low solubility of organic compounds and low substrate concentrations in traditional solvents such as water hinder its wider application in organic synthesis. A perfect solvent for biotransformations should be non-toxic, biocompatible, biodegradable, sustainable, and capable of supporting high enzyme activity and stability. Non-conventional solvents can deeply affect the stability, activity, and stereoselectivity of enzymes.
Cristina Prandi, University of Torino, Italy, and colleagues have developed the first enantioselective reduction of 2-substituted cyclic imines to the corresponding amines (pyrrolidines, piperidines, and azepines) by imine reductases (IREDs) in non-conventional solvents. The team used 5-phenyl-3,4-dihydro-2H-pyrrole as the model imine substrate and tested both, biomass-derived glycerol solvent/phosphate buffer and deep eutectic solvents (DES)/phosphate buffer mixtures. Glycerol is considered a green solvent because it comes from biomass sources and is a low-cost by-product of the biodiesel industry, making it an eco-friendly choice compared to volatile organic compounds (VOCs) in organic synthesis. The team also demonstrated that glucose-containing DES (ChCl/D-glucose) is a valid alternative, containing the necessary sugar for the redox cycle within the medium itself.
They achieved the most favorable outcomes in a 1:1 glycerol/phosphate buffer mixture, yielding heterocyclic amines with complete conversions (>99 %), satisfactory to excellent yields (22–84 %), and very good S-enantioselectivities (>99 % ee). This process can be performed at 100 mM substrate loading and up to 200 mM with a slight decrease in yield.
Additionally, they have developed a fed-batch procedure for convenient scale-up, resulting in the transformation of one millimole of substrate (5-phenyl-3,4-dihydro-2H-pyrrole) into 120 mg of the corresponding enantiopure (S)-amine with 80 % overall yield. The procedure uses a single batch of enzymes (IRED, NADP, and GDH-101), which allows a formal recycling of the catalytic system. This makes the process potentially attractive for large-scale applications for a specific set of substrates, leading to the production of enantiopure heterocyclic amines of high pharmaceutical interest.
- Asymmetric Reduction of Cyclic Imines by Imine Reductase Enzyme in Non‐conventional Solvents,
Davide Arnodo, Federica De Nardi, Stefano Parisotto, Eugenio De Nardo, Stefania Canana, Federica Salvatico, Elisa De Marchi, Dina Scarpi, Marco Blangetti, Ernesto Giovanni Occhiato, Cristina Prandi,
ChemSusChem 2023.
https://doi.org/10.1002/cssc.202301243