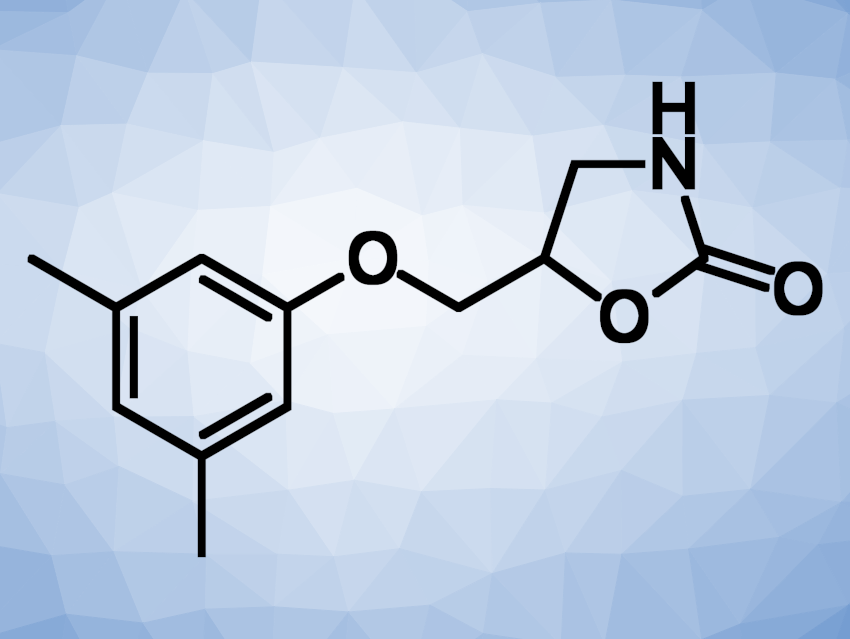
Metaxalone (pictured) is a muscle relaxant that contains an oxazolidinone unit. Many oxazolidinone derivatives are pharmaceutically active, and methods for their synthesis are interesting research targets. Halohydrin dehalogenases (HHDHs) can catalyze ring-opening reactions of epoxides in the presence of anionic nucleophiles. This could be useful for the synthesis of oxazolidinones when cyanate is used as the nucleophile and the epoxide opening is followed by a spontaneous cyclization reaction.
Yong-Zheng Chen, Nan-Wei Wan, Zunyi Medical University, China, and colleagues have developed a biocatalytic approach for synthesizing chiral or racemic metaxalone, as well as different metaxalone analogues. The team’s approach is based on protein engineering of a halohydrin dehalogenase from Acidimicrobiia bacterium (HHDHamb). They selected five residues in the enzyme as sites that could affect the binding of the reaction substrates and introduced mutations in these positions.
The researchers found that some of the generated mutant enzymes showed improved catalytic efficiency and enantioselectivity compared with the wild-type enzyme. They used these improved enzymes for the synthesis of oxazolidinones from low-cost cyanate and commercially available epoxides. The team achieved a gram-scale synthesis of chiral metaxalone in 44 % yield and 98 % ee using a quadruple mutant enzyme, while a triple mutant gave racemic metaxalone in 81% yield. They also used the method for the synthesis of metaxalone analogues.
- Biocatalytic Synthesis of Metaxalone and Its Analogues,
Zhu-Xiang Wang, Hong-Kang Fu, Xin-Yu Da, Hui-Hui Wang, Bao-Dong Cui, Wen-Yong Han, Yong-Zheng Chen, Nan-Wei Wan,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01752